Cembrane type diterpenoids with anti-tumor activities and application thereof
An anti-tumor activity and anti-tumor drug technology, applied in the field of medicine, can solve the problems of few reports on chemical components and biological activities, and achieve the effect of low toxicity and strong anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 cembrane type diterpene compound
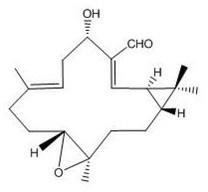
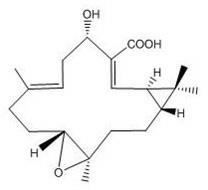
[0032] Take 20 kg of Euphorbia euphorbia root, crush it and extract it twice with 95% ethanol at 8 times the concentration for 2 hours each time, combine the extracts, recover the ethanol, and dry at low temperature to obtain the ethanol extract. After the ethanol extract was suspended in water, it was extracted three times with 1:1 petroleum ether, and the petroleum ether was recovered to obtain the petroleum ether fraction. Use silica gel column chromatography for the petroleum ether part, first elute with petroleum ether: ethyl acetate = 10: 1 for 10 column volumes, then elute with petroleum ether: ethyl acetate = 3: 1, combine petroleum ether: ethyl acetate = 3:1 elution site, repeated column chromatography to obtain pekinenins D (yield: 36.0 mg), the structural formula is as follows figure 1 As shown, and pekineninss E (yield: 28.0 mg), the structural formula is as figure 2 shown.
[0033] Stru...
Embodiment 2
[0035] Example 2 In vitro anti-tumor test
[0036] 1. Anti-human gastric cancer cell MGC-803 experiment
[0037] Human gastric cancer cell line MGC-803 was routinely cultured in RPMI-1640 medium containing 10% calf serum, 100 U / mL penicillin, and 0.1 mg / ml streptomycin in a 37°C, 5% CO2 incubator. Digest and passage with 0.25% trypsin plus 0.02% EDTA. Cells in the logarithmic growth phase were taken, digested with trypsin, and prepared cell suspension with RPMI-1640 culture medium containing 10% calf serum. Hole 180 μL; The new compound pekineninss D (structure such as figure 1 , the same below) and pekineninss E (structure such as figure 2 , the same below) were set at 6 concentrations of 1 μg / ml, 2 μg / ml, 5 μg / ml, 10 μg / ml, 20 μg / ml, and 40 μg / ml, and 20 μL dimethyl sulfoxide was added to each well, and 4 replicates were set in each group. Wells were cultured in a 37°C, 5% CO2 incubator for 72 hours, and 10 μL of WST-8 solution was added to each well. After continuing ...
Embodiment 3
[0056] Example 3 Acute toxicity test of pekineninss D and pekineninss E
[0057] Calculate the half lethal dose LD of mice according to Bliss method 50 value, LD 50 The value is 1.02 mg / kg, and the experimental results show that the pekineninss D and pekineninss E compounds provided by the present invention have relatively low acute toxicity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com