Trifluoromethyl-containing indole ketone compound, and preparation method and application thereof
A technology of trifluoromethyl compounds, which is applied in the field of trifluoromethyl-containing indolinone compounds and their preparation, can solve the problems of limited druggability, strong cytotoxicity, and poor stability of some compounds, and is easy to achieve Implementation and Scale, Operational Ease of Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
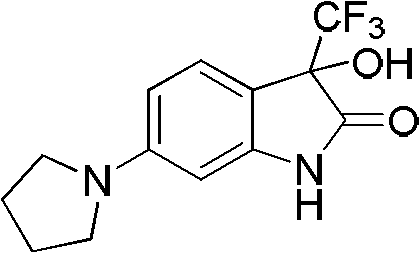
[0029] Example 1: Preparation of 3-hydroxyl-6-(1'-pyrrolidinyl)-3-trifluoromethylindol-2-one
[0030]
[0031] Take 3-pyrrolidinylaniline (365mg) and add 1mL of 1,2-dichlorobenzene, and slowly add ethyl trifluoropyruvate (0.3mL) dropwise at room temperature. After the addition, the reaction system is placed in a microwave reactor, and the power 500W, react at 150°C for 10 minutes, after the reaction is over, add a large amount of ethyl acetate to the system after cooling, extract, wash with water, wash with salt water, dry over anhydrous sodium sulfate, concentrate under reduced pressure and then purify by flash column chromatography to obtain the product. Yield 91%.
[0032] 1 H NMR (DMSO-d 6 , 400MHz) δ2.00-2.04(m, 4H), 3.28-3.31(m, 4H), 6.07(s, 1H), 6.17(d, J=2.0Hz, 1H), 6.23(dd, J=2.2, 8.2Hz, 1H), 7.25(d, J=8.8Hz, 1H), 9.46(brs, 1H). HRMS. Calculated value C1 3 h 13 f 3 N 2 o 2 : 286.0929. Measured value: 286.0926.
Embodiment 2
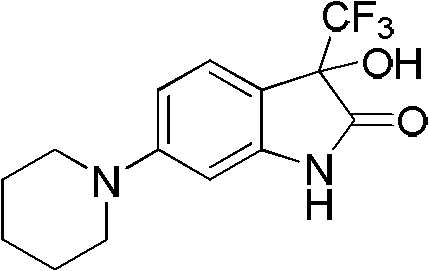
[0033] Example 2: Preparation of 3-hydroxyl-6-(1'-piperidinyl)-3-trifluoromethylindol-2-one
[0034]
[0035]Take 3-piperidinylaniline (397mg) and add 1mL of toluene, slowly add methyl trifluoropyruvate (0.3mL) dropwise at room temperature, after the addition, put the reaction system in a microwave reactor with a power of 500W, and react at 100°C After 10 minutes, the reaction was completed. After cooling, a large amount of ethyl acetate was added to the system, extracted, washed with water and brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure and purified by flash column chromatography to obtain the product with a yield of 94.5%. Mp 100.0~101.3℃;
[0036] 1 H NMR (Acetone-d 6 , 300MHz) δ1.50-1.56(m, 6H), 3.10-3.13(m, 4H), 6.07(s, 1H), 6.41(d, J=2.4Hz, 1H), 6.48(dd, J=2.4, 8.4Hz, 1H), 7.14(d, J=8.1Hz, 1H), 9.39(brs, 1H). HRMS. Calculated value C 14 h 15 f 3 N 2 o 2 : 300.1086. Measured value: 300.1084.
Embodiment 3
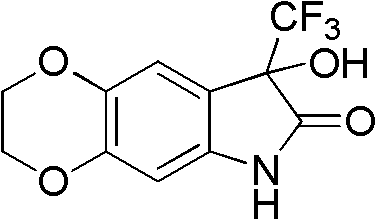
[0037] Example 3: Preparation of 8-hydroxyl-8-trifluoromethyl-6,8-dihydro-1,4-dimethylenedioxyindol-7-one
[0038]
[0039] Take 6-amino-1,4-benzodioxane (679.5 mg) and add 2 mL of chlorobenzene, slowly add methyl trifluoropyruvate (0.75 mL) dropwise at room temperature, and place the reaction system in a microwave reaction after the addition is complete. In the reactor, power 500W, react at 130°C for 15 minutes, after the reaction is over, add a large amount of ethyl acetate to the system after cooling, extract, wash with water, wash with salt water, dry over anhydrous sodium sulfate, concentrate under reduced pressure and then purify by rapid column chromatography to obtain an oil Product, the yield is 72%.
[0040] 1 HNMR (Acetone-d 6 , 300MHz) δ4.10-4.18(m, 4H), 6.25(s, 1H), 6.35(s, 1H), 6.84(s, 1H), 9.39(brs, 1H).
[0041] HRMS. Calculated value C 11 h 8 f 3 NO 4 : 275.0405. Measured value: 275.0402.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com