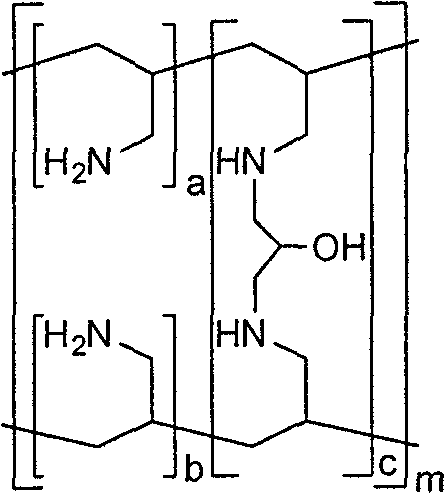
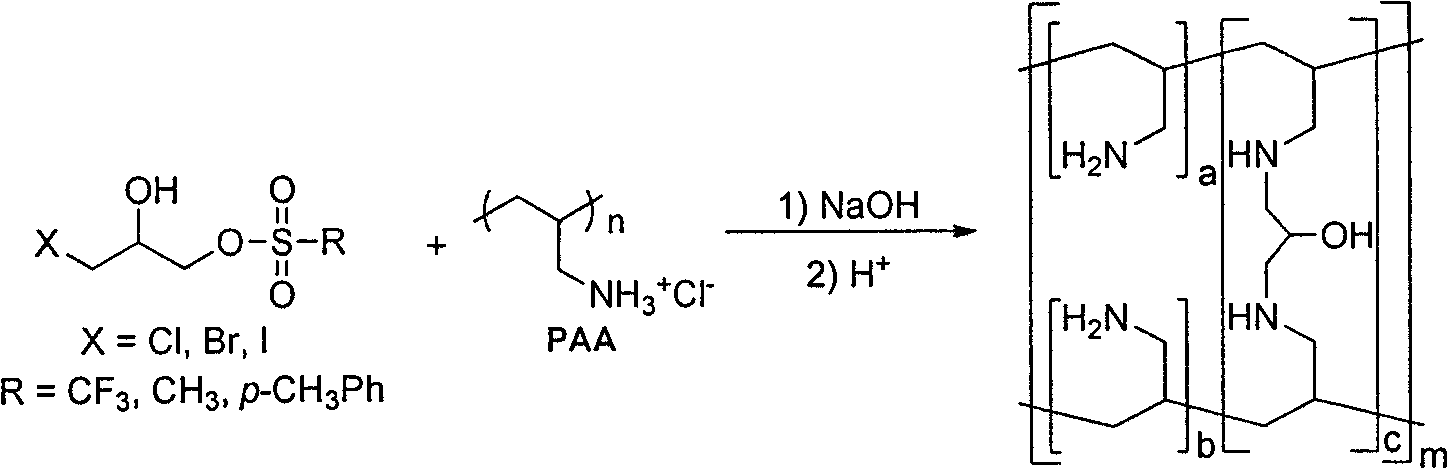
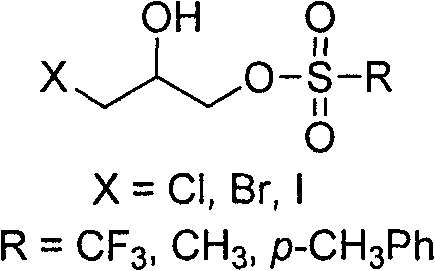Synthesis process of Sevelamer
A technique for the synthesis of Sevelamer hydrochloride, which is applied in the field of drug synthesis, can solve the problems of long reaction time and a large amount of organic solvents, and achieve the effects of shortened reaction time, simple operation, and strong phosphorus adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the synthesis of Sevelamer Hydrochloride
[0027] In a 500mL flask, add 46.2g (0.374mol) polyallylamine hydrochloride, dissolve in 108.0mL deionized water, add sodium hydroxide to adjust pH=10-11, add dropwise 11.0g (0.042mol) 1-chloro The toluene solution of generation-3-p-toluenesulfonyl-2-propanol was reacted at 70-75°C for 4 hours. After the reaction is complete, add hydrochloric acid to adjust the pH=1-2, and filter to obtain the crude product of sevelamer hydrochloride. Disperse the crude product of Sevelamer Hydrochloride in 300.0 mL of deionized water, add sodium hydroxide to adjust the pH=10.0-11.0, filter, wash with deionized water, and dry the white solid at 70°C for 8 hours in vacuum, then pulverize to obtain the product Sevelamer hydrochloride is 38.8g, the phosphorus adsorption value is 5.5mmol / g, and the chloride ion content is 16.5%.
Embodiment 2
[0028] Embodiment 2: the synthesis of carbonic acid sevelamer
[0029] In a 500mL flask, add 150.0g (0.404mol, weight concentration: 30%) polyallylamine hydrochloride aqueous solution, add sodium hydroxide to adjust pH=10-11, add dropwise 13.0g (0.042mol) 1-bromo Substitute-3-p-toluenesulfonyl-2-propanol in acetonitrile solution, heat up at 70-75°C for 4 hours. After the reaction is complete, add hydrochloric acid to adjust the pH=1-2, and filter to obtain the crude product of sevelamer hydrochloride. Disperse the crude product of sevelamer hydrochloride in 300.0mL deionized water, add sodium carbonate to adjust the pH=8.5-9.5, filter, wash with deionized water, and dry the white solid at 70°C for 8 hours in vacuum, and then pulverize it to obtain the product carbonic acid Sevelamer 36.4g, phosphorus adsorption value 5.4mmol / g.
Embodiment 3
[0030] Embodiment 3: the synthesis of carbonic acid sevelamer
[0031] In a 500mL flask, add 46.2g (0.374mol) polyallylamine hydrochloride, dissolve in 108.0mL deionized water, add sodium hydroxide to adjust pH=10-11, add dropwise 7.9g (0.042mol) 1-chloro Substitute-3-methylsulfonyl-2-propanol in toluene solution, heat up at 70-75°C for 4 hours. After the reaction is complete, add hydrochloric acid to adjust the pH=1-2, and filter to obtain the crude product of sevelamer hydrochloride. Disperse the crude product of sevelamer hydrochloride in 300.0 mL of deionized water, add sodium hydroxide to adjust the pH=12.0-12.5, pass in carbon dioxide gas to saturation, filter, wash with deionized water, and dry the white solid at 70°C for 8 hours in vacuum , and then pulverized to obtain 40.5 g of the product sevelamer carbonate, with a phosphorus adsorption value of 5.0 mmol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com