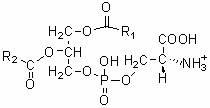Method for preparing phosphatidylserine
A technology of phosphatidylserine and serine, applied in fermentation and other directions, can solve the problems of low efficiency of enzymatic catalytic reaction and many by-products of phosphatidic acid, and achieve the effects of green process, improved enzymatic conversion efficiency and good product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
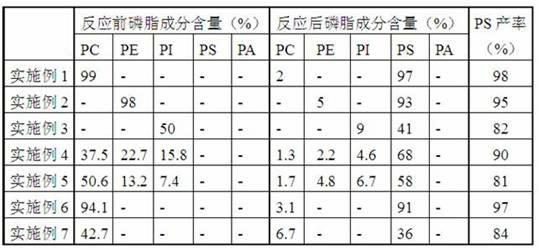
[0023] Phosphatidylcholine (99%, Sigma-Aldrich Company) and L-serine (commercially available) with a molar ratio of 1:9, and γ-valerolactone (Sigma-Aldrich Company) 5 times based on the mass of the reactant, Phospholipase D (derived from Streptomyces, Japan Asahi Kasei Co., Ltd.) based on 5% of the mass of the reactant was put into a biochemical reactor suitable for biological enzyme catalyzed reactions and mixed evenly, and the temperature was controlled at 65 o C, after 2 hours of reaction, the yield of phosphatidylserine reached 98%.
Embodiment 2
[0025] Phosphatidylethanolamine (98%, Sigma-Aldrich Company) and L-serine (commercially available) with a molar ratio of 1:6, and 2-methyltetrahydrofuran (Sigma-Aldrich Company) based on 1 times the mass of reactants, based on Phospholipase D (derived from Streptomyces, Asahi Kasei Co., Ltd., Japan) with 1% of the reactant mass was put into a biochemical reactor suitable for biological enzyme catalyzed reactions and mixed evenly, and the temperature was controlled at 45 o C, after 8 hours of reaction, the yield of phosphatidylserine reached 95%.
Embodiment 3
[0027] Phosphatidylinositol (50%, Sigma-Aldrich Company) and L-serine (commercially available) with a molar ratio of 1:3, and a mixture of γ-valerolactone and 2-methyltetrahydrofuran based on 8 times the mass of the reactant (Volume ratio 1:1, Sigma-Aldrich Company), phospholipase D (derived from Streptomyces, Sigma-Aldrich Company) based on the mass of reactants 10%, put together into any biochemical reactor suitable for biological enzyme-catalyzed reactions Mix well and keep the temperature at 35 o C, after 48 hours of reaction, the yield of phosphatidylserine reached 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com