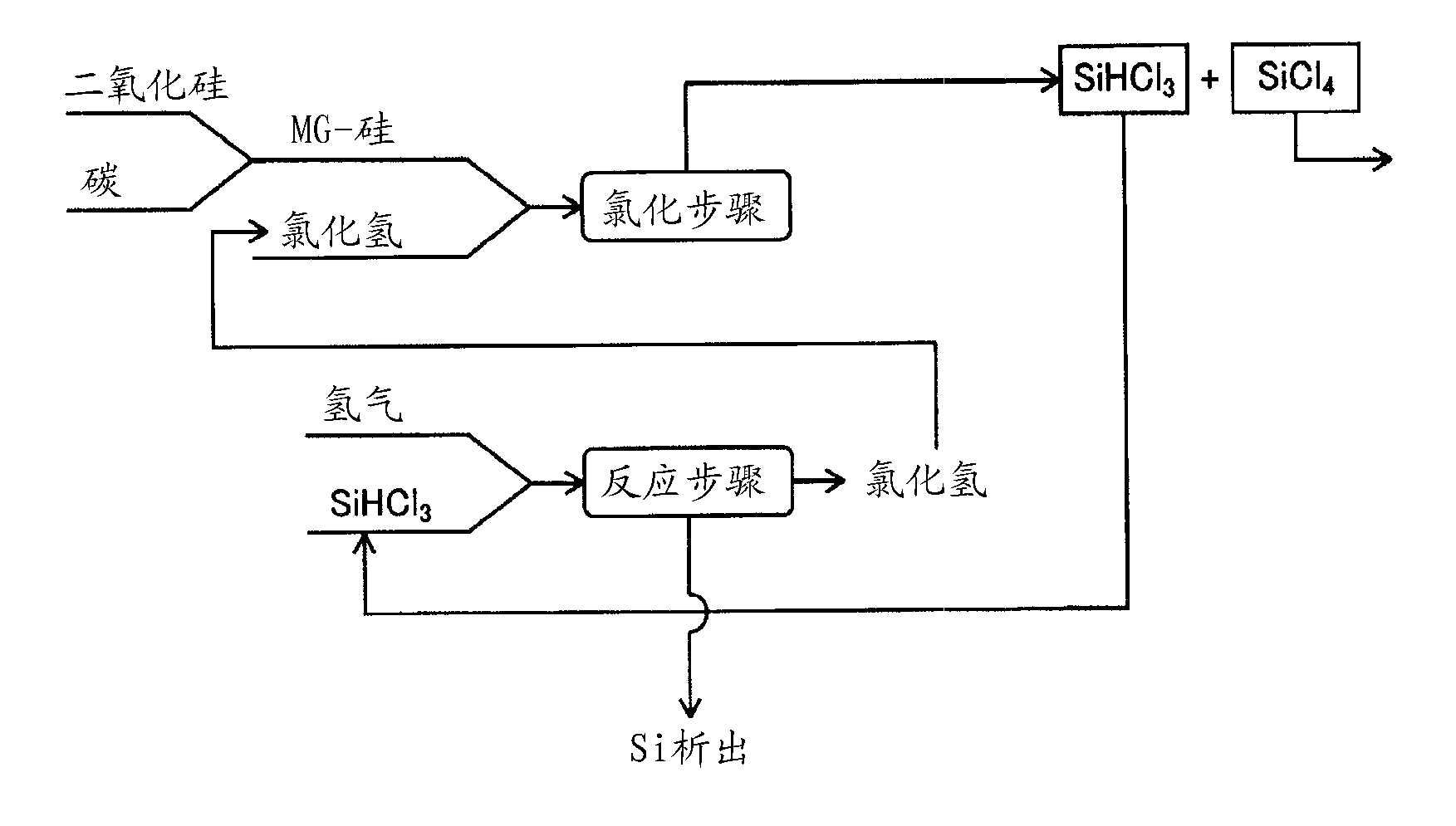
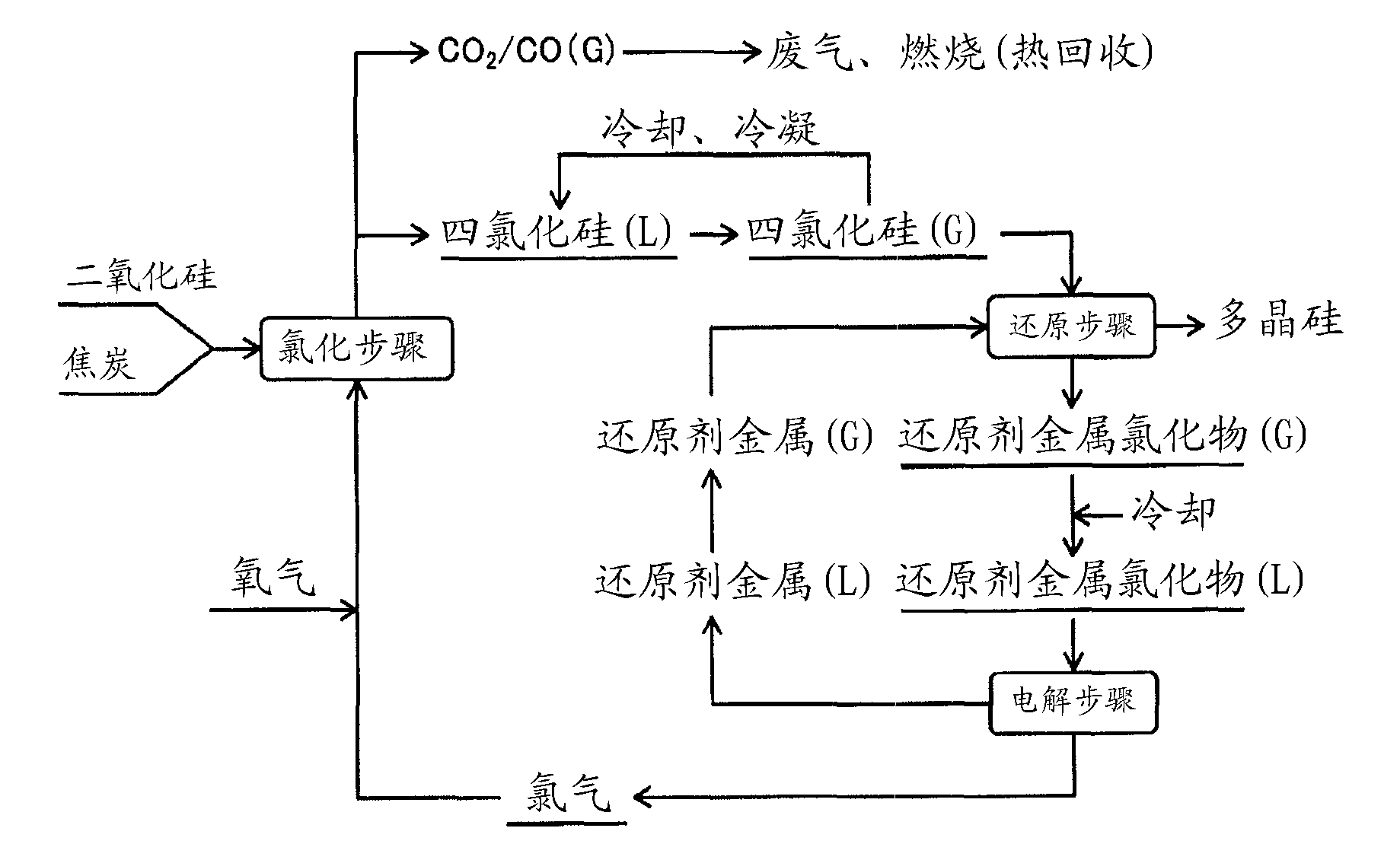
Method for manufacturing polysilicon and method for manufacturing silicon tetrachloride
A manufacturing method, a technology of silicon tetrachloride, applied in the direction of silicon compounds, silicon halide compounds, chemical instruments and methods, etc., can solve the problems of reduced yield of titanium tetrachloride and silicon tetrachloride, and achieve impurity Ingredient suppression, effective manufacturing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
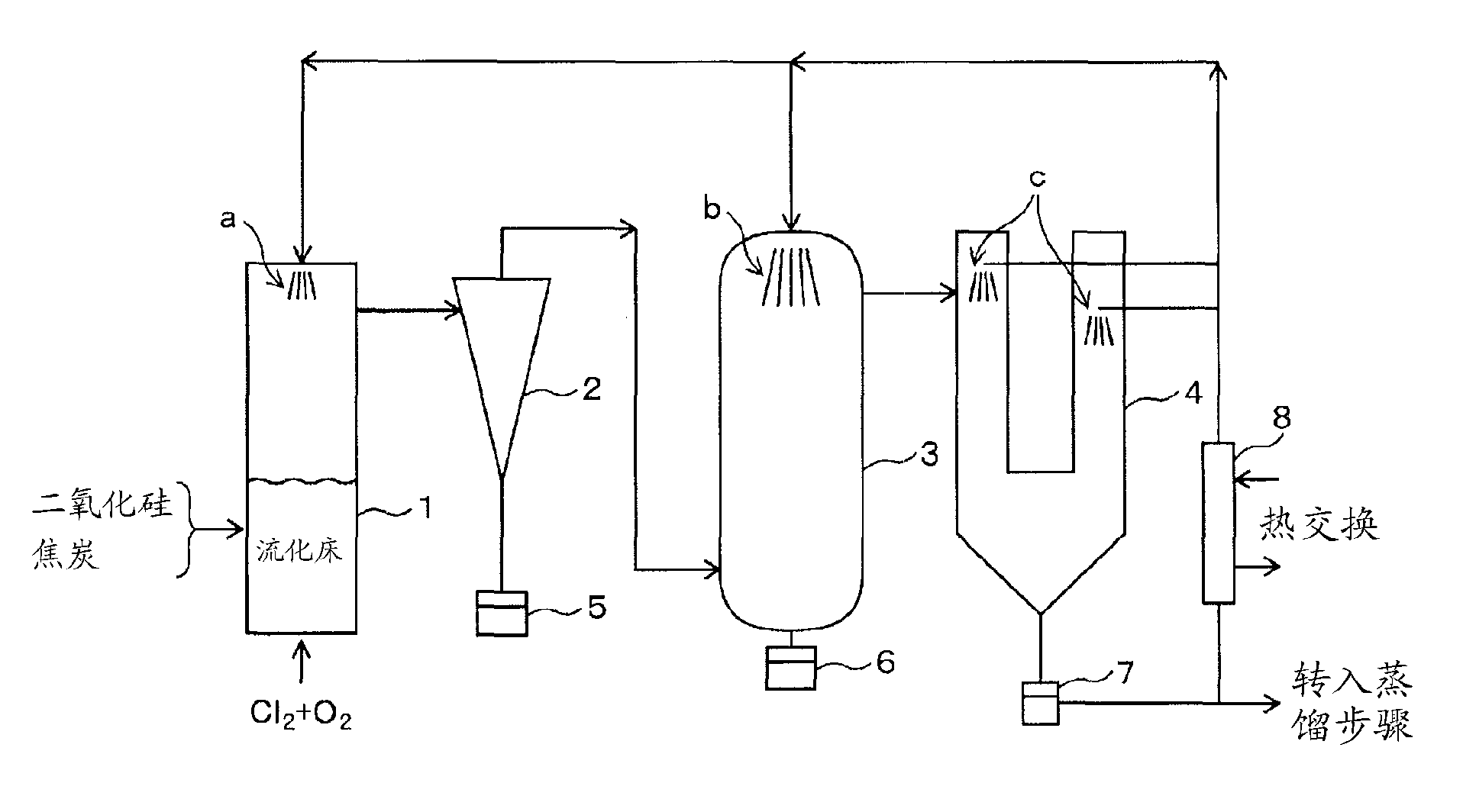
[0137] use figure 2 In the shown device, under the conditions shown below, in the chlorination step, silicon dioxide is used as a raw material to produce silicon tetrachloride, which is reduced with metal zinc vapor in the reduction step to produce solid polysilicon. In addition, zinc chloride, which is by-produced in the reduction reaction, is electrolyzed into metal zinc and chlorine gas in the electrolysis step, metal zinc is recycled as a reducing agent for silicon tetrachloride, and chlorine gas is recycled as a chlorinating agent for silicon dioxide . Furthermore, the polysilicon produced in the reduction step is melted, and high-purity silicon is deposited on the high-purity seed crystal.
[0138] 1. Chlorination step
[0139] 1) Raw material
[0140] The following raw materials were used to form granules of 1 to 2 mm for the chlorination reaction.
[0141] (1) Silica: purity 98wt%, particle size after pulverization 5μm
[0142] (2) Coke: purity 90wt%, particle ...
Embodiment 2
[0166] The silica and coke before pulverization in Example 1 were blended at a molar ratio of 1:2, put into a ball mill, pulverized by a pulverizer, and the particle diameters of silica and coke were changed by changing the pulverization time. Next, after adding 25% of TEOS to the silica and coke, granules were formed using a granulator. Then, after heating and drying the above-mentioned granules, the granules were sized to 0.5 mm to 1 mm, and then a chlorination test was performed using a fixed bed to confirm the production of silicon tetrachloride.
[0167] The reaction rate index of silica was calculated by the above formula (1), and the reaction rates confirmed under various test conditions are summarized in Table 1.
[0168] It was confirmed that silicon tetrachloride was efficiently produced by subjecting granules composed of silica having a particle diameter of 5 μm or less and coke having a particle diameter of 10 μm or less to the chlorination reaction. Among them, i...
Embodiment 3
[0172] In Example 2, various molar ratios of silica and coke constituting the granules were changed, and the influence on the production state of silicon tetrachloride was investigated. The results are shown in Table 2. When the molar ratio of coke to silica is 1.0 to 4.0, the utilization rate of chlorine gas is 90% or more, showing good reactivity. However, when the molar ratio of coke to silica was 0.5, the utilization rate of chlorine gas decreased to 50%.
[0173] Wherein, the utilization rate of chlorine is defined as the molar ratio (%) of the amount of chlorine calculated from the recovered silicon tetrachloride relative to the amount of input chlorine. It was confirmed from this Example that the molar ratio of coke constituting the granules to silica is preferably in the range of 1.0 to 4.0.
[0174] [Table 2]
[0175]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com