Application of nestorone to preparation of drugs for treating Parkinson's disease
A Parkinson's disease and drug technology, applied in the fields of neurology and pharmacology, can solve problems such as Nestorone and Parkinson's disease research reports that have not been seen, and achieve the effect of improving and treating curative effect, with less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Example 1: MPTP was used to establish a mouse model of Parkinson's disease, and Nestorone was injected subcutaneously for pretreatment before modeling and intervention after modeling
[0012] 84 C57 mice, weighing 18g-25g, were randomly divided into 7 groups. Normal control group (Con): subcutaneous injection of normal saline (0.01ml·g) twice a day -1 ) A total of 11 days, on the 6th day, another intraperitoneal injection of normal saline (0.01ml·g -1 ) 4 times; model group (Mod): subcutaneous injection of normal saline (0.01ml·g) twice a day -1 ) A total of 11 days, the 6th day of intraperitoneal injection of MPTP; solvent group (Veh): twice daily subcutaneous injection of 10% ethanol (0.01ml·g -1 ) A total of 11 days, the 6th day of intraperitoneal injection of MPTP; Nestorone low dose group (0.08mg·kg -1 ·D -1 , Nes L): Nestorone 0.04mg·kg subcutaneously injected twice daily -1 A total of 11 days, the 6th day of intraperitoneal injection of MPTP; Nestorone high-dose group...
Embodiment 2
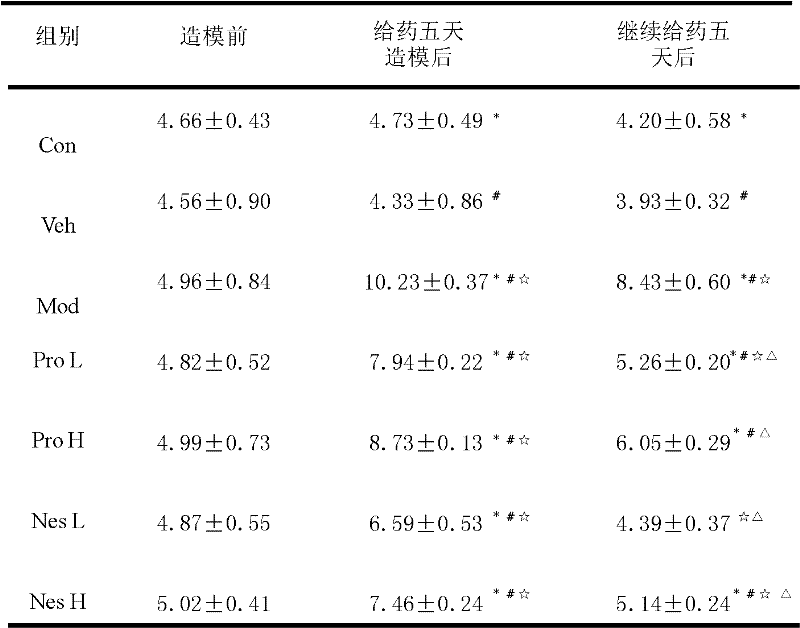
[0014] Example 2: Climbing pole experiment to detect the movement coordination ability of mice
[0015] A metal climbing rod with a height of 50 cm, a diameter of 1 cm, and a rough surface were made before the model, five days after the injection, and five days after the drug. The rod was vertically fixed on the base. The top of the rod was a small wooden ball with a diameter of 2.5 cm. The rod and ball are tightly wrapped with gauze to prevent the mice from slipping. According to the method described therein and improved, the time is measured 1 day before administration, 1 day after MPTP administration, and 1 day after the end of administration. The time is measured by placing the mouse's head horizontally on the ball on the top of the rod, and the time from the forelimbs beginning to touch the rod body until the forelimbs touch the ground. .
[0016] The results showed that after modeling, the climbing time of the Mod group and the Veh group was significantly longer than that of...
Embodiment 3
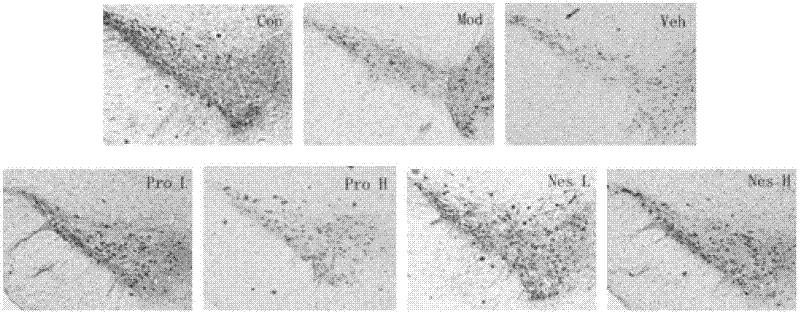
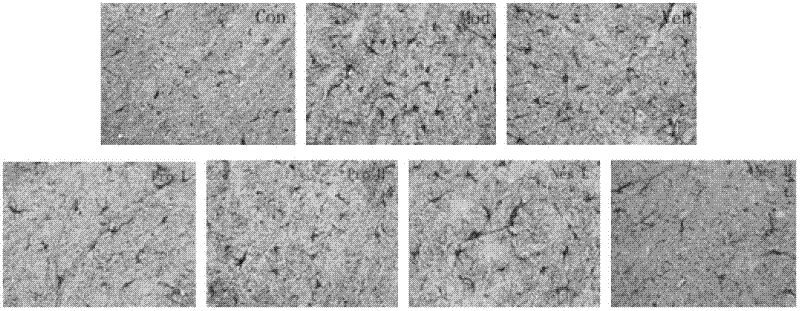
[0020] Example 3: Detecting the expression of TH and GFAP in the substantia nigra of mice by immunohistochemistry
[0021] First, give the model according to Example 4, and after the pole climbing test on the 12th day according to Example 5, intraperitoneal injection of 0.01ml·g -1 Mice in each group were anesthetized with 8% (W / V) chloral hydrate. Six mice were randomly selected to open the chest after the consciousness disappeared, and the left ventricle-aorta was intubated, and the mice were perfused with normal saline and 4% paraformaldehyde (4℃). If the mice’s liver was white and the limbs were stiff, the perfusion was successful. The brain was immersed in 4% paraformaldehyde and fixed, embedded in paraffin, and sectioned. After routine deparaffinization of brain tissue sections in water, immersion in PBS; high pressure heat repair of tissue antigen in citric acid repair solution; PBS washing; 3% hydrogen peroxide blocking for 20 minutes; PBS washing; adding TH antibody (1:1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com