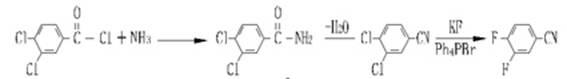
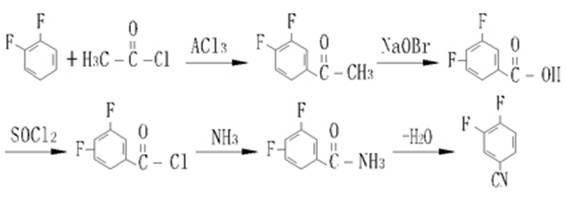
Industrial preparation process for 3,4-difluorobenzonitrile
A technology of difluorobenzonitrile and preparation process, applied in 3 fields, can solve the problems of inability to meet industrial production needs, strict reaction requirements, complex preparation process, etc., and achieves the possibility of reducing fluorine loss and coking, short reaction process flow, and improved performance. Effect of Reaction Yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Add 456Kg solvent and 150L toluene from solvent DMI head tank and toluene head tank respectively to 2000L fluorination kettle with rectification tower;
[0032] 2. Turn on the fluorination kettle to stir, open the feeding port and put 139.2Kg of anhydrous potassium fluoride and 20Kg of catalyst into the fluorination kettle, close the feeding port, and check whether the seal is intact;
[0033] 3. Turn on the vacuum system so that the gauge pressure of the fluorination kettle is -0.1Mpa. After the vacuum is stable, open the cooling water system of the top condenser of the fluorination kettle, start the hot oil pump, and slowly raise the temperature; when the temperature in the kettle rises to 110°C , the rectifying tower is in the total reflux state, and the discharge flow rate is controlled at 55kg / h, the discharge ends, the clear cut toluene is finished, and the discharge valve is closed so that the tower is in the total reflux state;
[0034] 4. Open the vent valve...
Embodiment 2
[0040] 1. Add 342Kg solvent and 150L toluene from the solvent DMI head tank and toluene head tank respectively to the 2000L fluorination kettle with rectification tower;
[0041] 2. Turn on the fluorination kettle to stir, open the feeding port and put 127.6Kg of anhydrous potassium fluoride and 12.5Kg of catalyst into the fluorination kettle, close the feeding port, and check whether the seal is intact;
[0042] 3. Turn on the vacuum system so that the gauge pressure of the fluorination kettle is -0.1Mpa. After the vacuum is stable, open the cooling water system of the top condenser of the fluorination kettle, start the hot oil pump, and slowly raise the temperature; when the temperature in the kettle rises to 110°C , the rectifying tower is in the total reflux state, and the discharge flow rate is controlled at 55kg / h, the discharge ends, the clear cut toluene is finished, and the discharge valve is closed so that the tower is in the total reflux state;
[0043]4. Open the v...
Embodiment 3
[0049] 1. Add 912Kg solvent and 150L toluene from solvent DMI head tank and toluene head tank respectively to 2000L fluorination kettle with rectification tower;
[0050] 2. Turn on the fluorination kettle to stir, open the feeding port and put 232Kg of anhydrous potassium fluoride and 25Kg of catalyst into the fluorination kettle, close the feeding port, and check whether the seal is intact;
[0051] 3. Turn on the vacuum system so that the gauge pressure of the fluorination kettle is -0.1Mpa. After the vacuum is stable, open the cooling water system of the top condenser of the fluorination kettle, start the hot oil pump, and slowly raise the temperature; when the temperature in the kettle rises to 110°C , the rectifying tower is in the total reflux state, and the discharge flow rate is controlled at 55kg / h, the discharge ends, the clear cut toluene is finished, and the discharge valve is closed so that the tower is in the total reflux state;
[0052] 4. Open the vent valve t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com