Synthesis method of diglycidyl 4,5-epoxycyclohexane-1,2-dicarboxylate
A technology of glycidyl ester and diformic acid, which is applied in the field of preparation of organic epoxy compounds, can solve the problems of serious equipment corrosion, complicated reaction process and high production cost, achieve high conversion rate, short process and improve epoxy value. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] Preparation of Phosphotungstic Heteropolyacid Quaternary Ammonium Salt Catalyst:
[0025] Take hexadecyltrimethylammonium bromide as an example to prepare phosphotungstic heteropolyacid quaternary ammonium salt catalyst:
[0026] Add 20g of tungstic acid (80mmol) and 85ml of 30% hydrogen peroxide (832.5mmol) aqueous solution into the reactor, stir and heat to 60°C, react for 80 minutes, cool to room temperature, and add 2.32g Phosphoric acid (20mmol) with a percentage concentration of 85% was dissolved in 9ml of water and added to the reaction solution, and the reaction solution was diluted with 180ml of distilled water and stirred for 50 minutes; 14.6 grams of cetyltrimethylammonium bromide (40mmol) was dissolved In 500ml of chloroform, add dropwise to the above reaction solution, and continue to stir for 80 minutes, static layering, separate the organic phase and distill under reduced pressure to remove the solvent in the organic phase, and the residue is heated at 6...
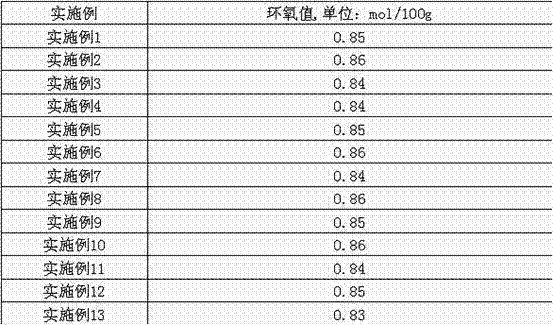
Embodiment 1
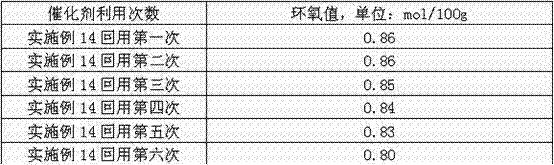
[0030] In a 2000ml four-necked flask, add 564g 1,2-dichloroethane, 564g (2.0mol) diglycidyl tetrahydrophthalate, 10g (5.3×10 -3 mol) Catalyst B, 0.6 g (1.68×10 -3 mol) disodium hydrogen phosphate buffer, first add 50% by weight aqueous hydrogen peroxide solution (50% by weight concentration, 1.1mol of pure hydrogen peroxide) 74.8g into the reaction flask under stirring, and make the reaction Keep the temperature in the container at 45-50°C, stir and react for 1 hour, add the remaining 50% aqueous hydrogen peroxide solution, continue to react at 45-50°C for 5 hours, after the reaction is completed, cool the material to room temperature, and let it stand For 2 hours, separate the organic phase and the water phase and remove the water, filter the organic phase to obtain a filter cake, and obtain the recovered quaternary ammonium salt catalyst of phosphotungstic heteropolyacid, then distill the organic filtrate under reduced pressure or atmospheric pressure to recover the solvent,...
Embodiment 2
[0032] Add 1692g methylene chloride, 846g (3.0mol) diglycidyl tetrahydrophthalate, 29.1g (1.5×10 -2 mol) Catalyst A, 0.58 g (3.34×10 -3 mol) dipotassium hydrogen phosphate buffer, first add 50% of the total weight of hydrogen peroxide aqueous solution (50% by weight concentration, 3.0mol of pure hydrogen peroxide) into the reaction bottle under stirring, at 30 ~ 35 ℃ After stirring and reacting at high temperature for 2 hours, add the remaining half of 204g hydrogen peroxide aqueous solution into the reactor, and continue to react for 12 hours at 30-35°C after the addition is completed. After the reaction is completed, cool the material to room temperature and let it stand 8 hours, other with embodiment 1, omission.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com