Au/TiO2 nanocrystalline catalysts and application thereof
A nano-catalyst and nano-crystal technology, applied in the field of nano-materials, can solve the problems of low conversion rate of propylene oxide, low rate of propylene oxide, environmental pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
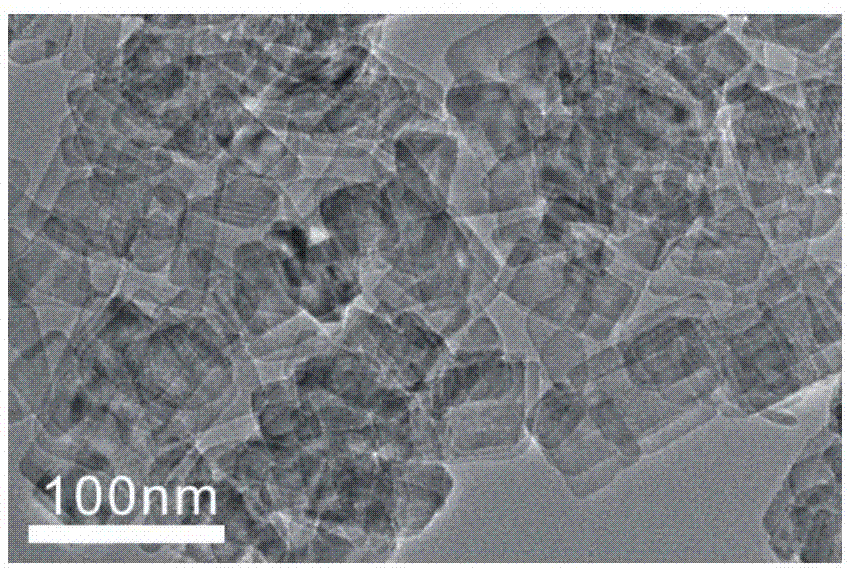
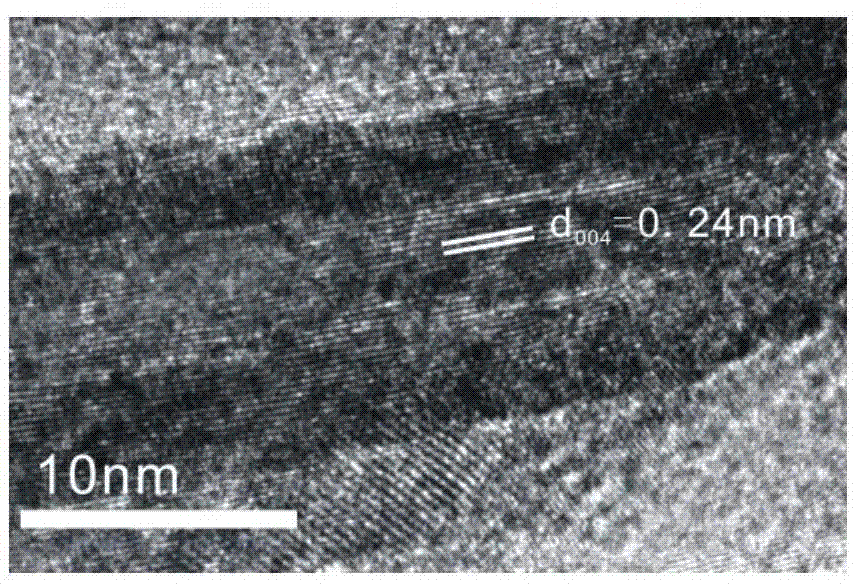
[0029] The main exposed (001) surface of TiO 2 (001) Preparation of nanocrystals:
[0030] Take 25ml of tetra-n-butyl titanate and 3ml of hydrofluoric acid in 50ml of polytetrafluoroethylene inner village and stir at room temperature for half an hour, then put it into a stainless steel jacket, keep it at 180°C for 24h, and centrifuge the obtained product after the reaction , washed repeatedly with ethanol and ultrapure water to obtain white TiO 2 (001) solid powder, and dried at 80°C for 12 hours, and then washed with 700ml 0.1M NaOH to obtain TiO 2 (001) solid powder, and then repeatedly washed with ethanol and ultrapure water, and finally a clean white TiO 2 (001) Solid powder.
[0031] Preparation of TiO as above 2 The method of (001) nanocrystals refers to the method provided by Xie Zhaoxiong et al. Materials such as tetra-n-butyl titanate, hydrofluoric acid, absolute ethanol and ultrapure water used in the preparation process are all purchased from the market.
Embodiment 2
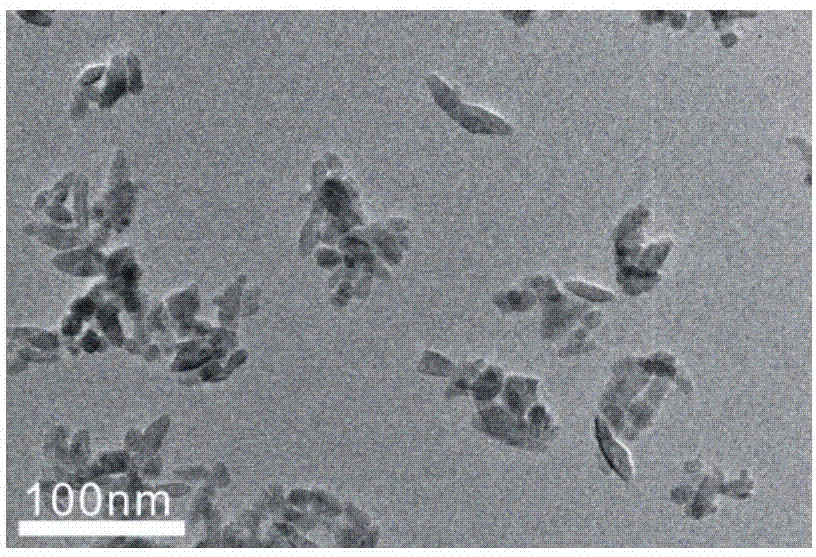
[0033] The main exposed (100) facet of TiO 2 (100) Preparation of nanocrystals:
[0034] First synthesize Ti(OH) 4 , first prepare 0.43mol / L HCl solution, 5.5% and 4% ammonia solution by mass fraction, then take 20ml HCl solution in ice-water bath, then add 6.6ml TiCl dropwise 4 , to obtain clarified TiCl 4 solution. These clarified TiCl 4 The solution was added dropwise to 50ml of 5.5% ammonia solution to produce a white precipitate, and the pH of the reactant was adjusted to 6-7 with 4% ammonia solution. Then it was stirred for 2 h, and the resulting white precipitate was washed with ultrapure water until the AgNO 3 Solution detection No Cl in filtrate -1 . Then vacuum dry at 60°C for 12 hours to obtain white Ti(OH) 4 .
[0035] Using Ti(OH) 4 as a precursor to synthesize TiO 2 (100), 0.5g (NH 4 ) 2 SO 4 Dissolve in 15ml ultrapure water and 15ml isopropanol mixture, add 2g Ti(OH) 4 , and put it into a 50ml polytetrafluoroethylene inner village and stir at room...
Embodiment 3
[0038] Au / TiO loaded with Au mass fraction of 1% 2 (001) Preparation of nanocrystalline catalyst, and its activity in the reaction of propene epoxidation to propylene oxide:
[0039] With the TiO that 1g embodiment 1 makes 2 (001) nanocrystals are placed in a three-necked flask, then add 50ml of deionized water, and add Au and TiO 2 A gold precursor solution with a mass ratio of 1%, adjust the pH of the solution to 7-10, and stir at 60° C. for 1 h. Then the obtained product was filtered and washed until the filtrate was washed with AgNO 3 Solution until no precipitate can be detected. Finally, dry at 60°C for 12h in vacuum, and bake at 400°C for 2h in an air atmosphere.
[0040] The relevant catalytic performance test is done to the product obtained in the present embodiment below:
[0041] The propylene epoxidation reaction is carried out in a quartz reaction tube. 250mg of catalyst is added to the quartz reaction tube with quartz wool. The gas used is a mixture of propy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com