Clofarabine injection and preparation method thereof
A clofarabine and injection technology, applied in the field of clofarabine injection and its preparation, can solve the problems of low stability, unsuitability for storage, low solubility and the like of freeze-dried products, so as to reduce the cost of medication and reduce the amount of infusion , good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
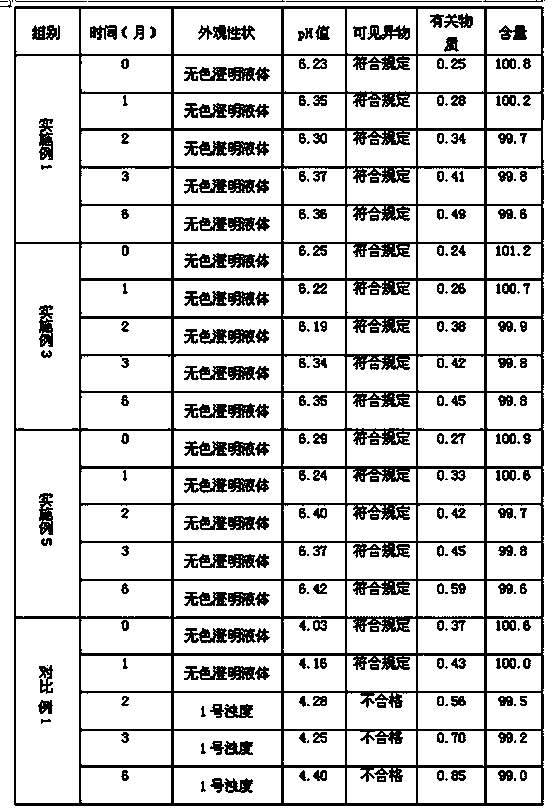
Examples
preparation example Construction
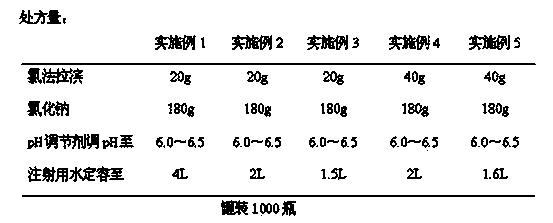
[0027] The preparation technology of above-mentioned embodiment is as follows:
[0028] (1) Weigh sodium chloride, add water for injection with 90% of the total volume of the prescription amount, add activated carbon for injection with 0.1% of the total weight of water for injection, stir and decarbonize to obtain a medicinal solution;
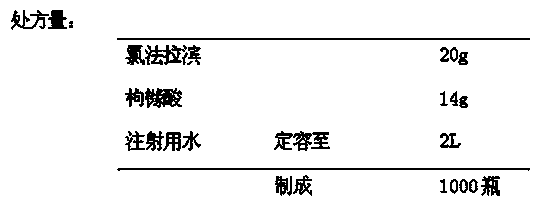
[0029] (2) Take citric acid aqueous solution, add sodium hydroxide solution to adjust the pH value to 6.2, and obtain citrate buffer solution;
[0030] (3) Add clofarabine to the medicinal solution obtained in step (1), heat to 80°C, stir evenly, add the citrate buffer solution prepared in step (2), and adjust the pH of the medicinal solution to 6.0~ 6.5, add the remaining amount of water for injection to make up the volume;
[0031] (4) Filter the solution obtained in step (3) through a 0.22um microporous membrane, fill it, press the plug, cover it, and sterilize it with damp heat to obtain the finished product. Sterilization conditions for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com