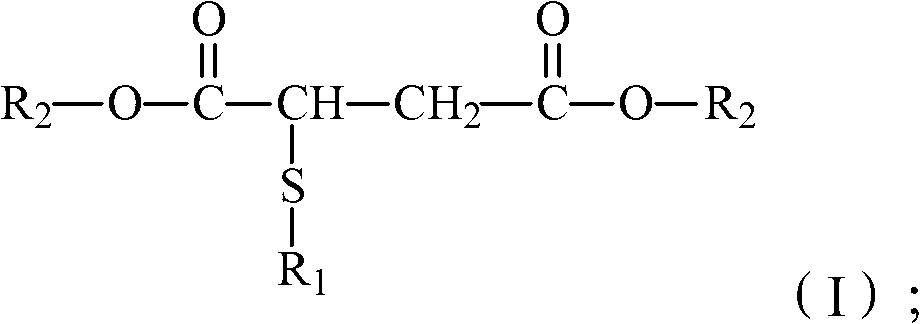Ester compound, preparation method thereof and plastic product
A technology of ester compounds and compounds, which is applied in the field of plastic products and ester compounds with the structure of formula (I), can solve the problems of environmental and human harm, poor performance of plastic products, poor low temperature resistance at freezing point, etc., and achieve benefits Effects of application, increased softness and elasticity, and good low temperature resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The present invention also provides a method for preparing an ester compound having a structure of formula (I), comprising the following steps:
[0037] Under the action of a catalyst and water, react the compound with the structure of formula (II) with the monohydric alcohol with the structure of formula (III) to obtain the ester compound with the structure of formula (I);
[0038]
[0039] R 2 -OH(III);
[0040] Among them, R 1 Selected from C4~C12 alkyl, C7~C14 aralkyl or C7~C14 alkaryl; R 2 Alkyl group selected from C3~C10 or aralkyl group of C9~C16.
[0041] The present invention uses the compound with the structure of formula (II) and monohydric alcohol with the structure of formula (III) as raw materials to prepare the ester compound with the structure of formula (I). When it is used as a cold-resistant plasticizer, the Low temperature performance is better.
[0042] In the present invention, the compound with the structure of formula (II), the monohydric...
Embodiment 1
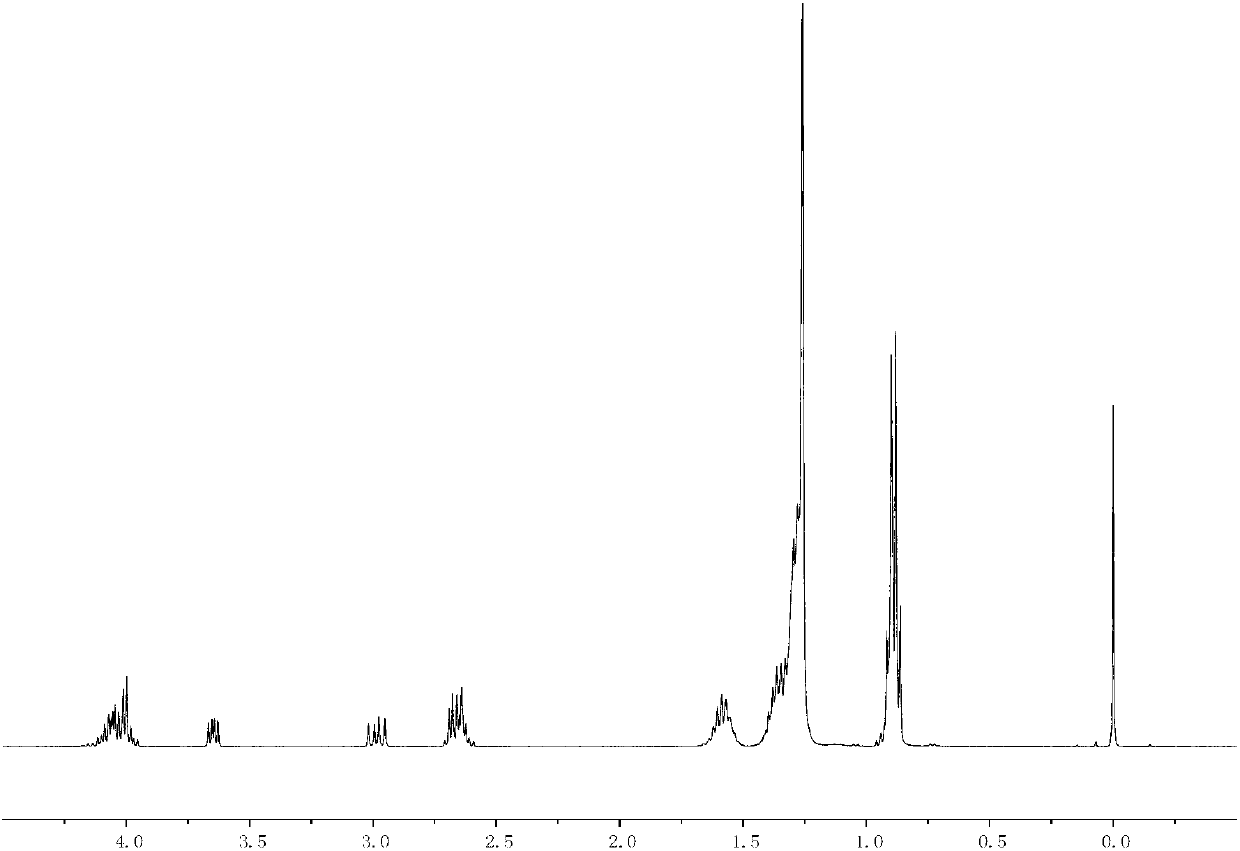
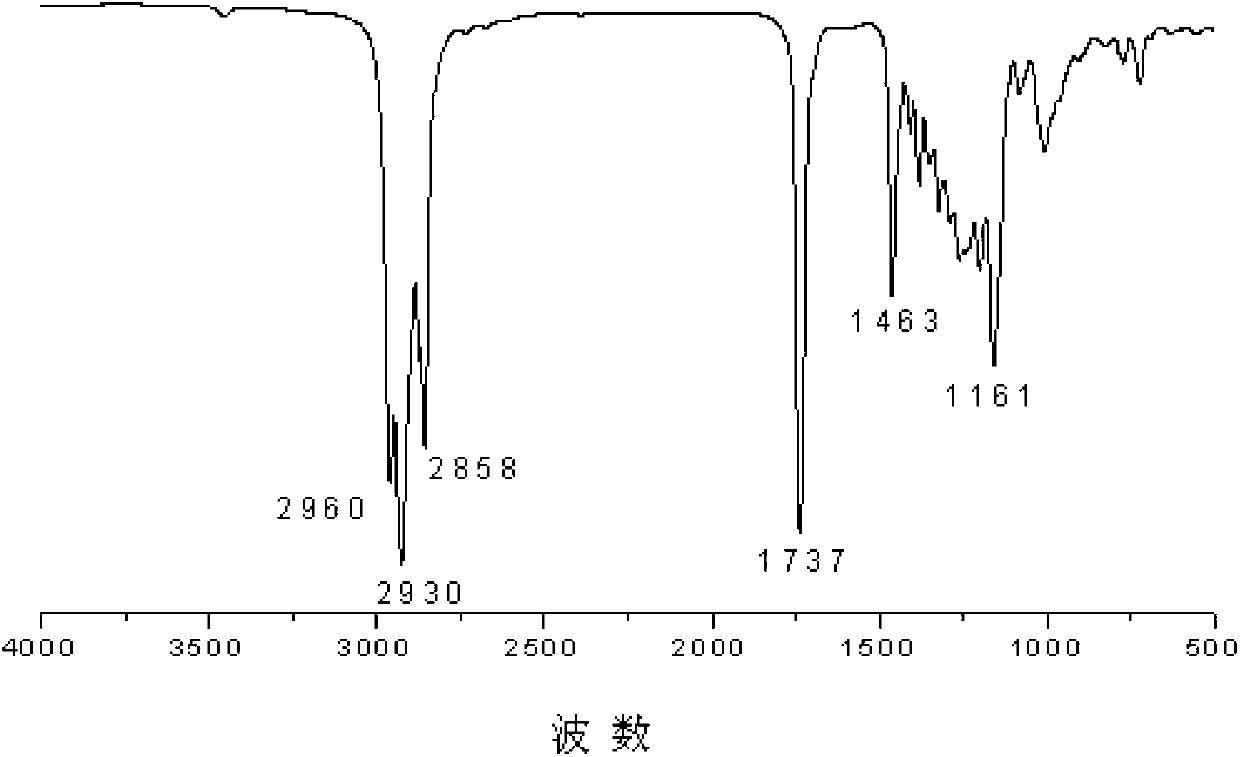
[0075] Add 19.6g maleic anhydride and 200mL tetrahydrofuran to the reactor, add 0.25mL triethylamine dropwise and perform electromagnetic stirring, then add 40.2g n-dodecylmercaptan dropwise into the reactor at 25°C to replace After the reaction was stirred for 3h, the substitution reaction was completed.
[0076] After the completion of the substitution reaction, the tetrahydrofuran was removed by rotary evaporation, then recrystallized with petroleum ether, filtered and dried to obtain a compound with the structure of formula (II), R 1 For n-dodecyl.
[0077] Add 30g of the obtained compound with the structure of formula (II), 50g of isooctyl alcohol and 0.5g of n-butyl titanate into an unbaked three-necked flask, and react under reflux with water at 140°C under the condition of mechanical stirring , The reaction was completed after 4h.
[0078] After the reaction was completed, excess isooctyl alcohol was removed by distillation under reduced pressure, dissolved with indu...
Embodiment 2
[0087] Add 19.6g maleic anhydride and 200mL tetrahydrofuran to the reactor, add 0.25mL triethylamine dropwise and perform electromagnetic stirring, then add 21.9g thiophenol dropwise into the reactor at 25°C for substitution reaction, and stir for 3h After that, the substitution reaction is completed.
[0088] After the completion of the substitution reaction, the tetrahydrofuran was removed by rotary evaporation, then recrystallized with petroleum ether, filtered and dried to obtain a compound with the structure of formula (II), R 1 For phenyl.
[0089] Add 21g of the obtained compound with the structure of formula (II), 40g of isopropanol and 0.5g of n-butyl titanate into an unbaked three-necked flask, and react under reflux with water at 170°C under the condition of mechanical stirring , The reaction was completed after 4h.
[0090] After the reaction was completed, excess isopropanol was removed by distillation under reduced pressure, dissolved with industrial ethanol af...
PUM
| Property | Measurement | Unit |
|---|---|---|
| freezing point | aaaaa | aaaaa |
| freezing point | aaaaa | aaaaa |
| freezing point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com