shRNA lentiviral expression vector for specifically inhibiting hepatic cell CYP2E1 gene expression, constructing method and application thereof
A CYP2E1, expression vector technology, applied in the field of genetic engineering, can solve the problems of inability to achieve long-term stable interference, insufficient interference effect, low transfection efficiency, etc., and achieves good inhibition effect, good operation effect, and high transfection efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Design of shRNA oligonucleotide sequences targeting hepatocyte CYP2E1 gene.
[0039] The complete mRNA sequence of CYP2E1 (NM_000773.3) was found in GenBank, and the specificity was confirmed by BLAST homology comparison, and the secondary structure of the target mRNA sequence was evaluated by using RNA structure 4.4 software, and finally three 19nt target nuclei were screened out Nucleotide sequence, the specific sequence is shown in Table 1.
[0040]
[0041] According to the target nucleotide sequence, the DNA template single strands of two shRNAs are designed and synthesized. The design is as follows: Age I restriction site + 19 nt target nucleotide sequence + stem-loop structure (TTCAAGAGA) + target sequence complementary sequence + RNA Poly III polymerase transcription termination site (TTTTTT) + EcoRI restriction site six regions, They were named shRNA1, shRNA2, and shRNA3, respectively, and a pair of control sequences siRNAc was designed. The spe...
Embodiment 2
[0043] Example 2 Construction of shRNA lentiviral expression vector.
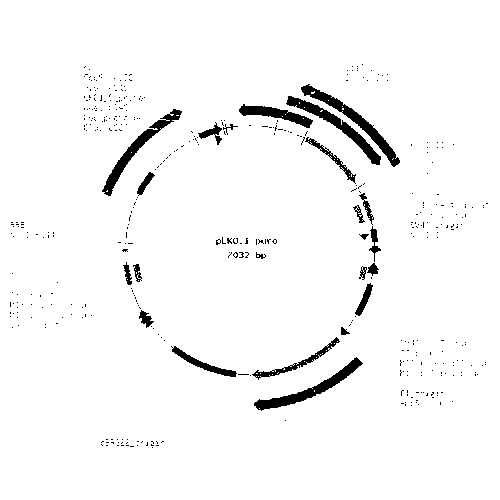
[0044] Mix the synthetic shRNA oligonucleotide single strands in equal amounts, anneal to form double-stranded shRNA, and extract the plasmid PLKO.1-puro (map as figure 1 shown), double-digested with restriction endonucleases AgeⅠ and EcoRI, recovered the vector by electrophoresis and gel cutting, and then ligated the double-stranded shRNA1, shRNA2, shRNA3, and shRNAc into the vector PLKO.1-puro with T4 DNA ligase, respectively. The recombinant plasmids pLKO-2E1shRNA1, pLKO-2E1shRNA2, pLKO-2E1shRNA3, pLKO-2E1shRNAc were formed. Transform the ligation product into competent Escherichia coli JM107, smear it on a plate containing ampicillin LB medium, and incubate at 37°C for 14 hours, and set a negative control group 1 (uniformly spread competent cells on a plate without ampicillin). plate), negative control group 2 (uniformly spread competent cells on a plate containing 100 μg / ml ampicillin), positive con...
Embodiment 3
[0047] Example 3 Lentiviral packaging.
[0048] 293FT cells were cultured, and the cells in good growth state were inoculated into six-well plates, 10 per well 6 2 μg of the extracted recombinant plasmids pLKO-2E1shRNA1, pLKO-2E1shRNA2, pLKO-2E1shRNA3, and pLKO-2E1shRNAc were respectively mixed with 1 μg of lentiviral packaging plasmids pCMV-VSV-G and 2 μg of pCMV-dR8.91 by using Lipofectamine® 2000. Transfect into 293FT cells, collect virus-containing supernatant medium at 48h and 72h respectively, filter the virus liquid with a 0.45 μm sieve, and use it to transduce L-02 liver cells. The titer of the virus detected by the Lenti-X GoStix kit is 5×10 6 ~5×10 7 IFU.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com