Analytical determining method for chemical phase of vallerite in copper sulfide ore
A determination method and technology for chemical substances, which are applied in the direction of analysis by chemical reaction of materials and material analysis by observing the influence of chemical indicators, can solve the problems of long process, large error, low efficiency, etc. The effect of large error and improved accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
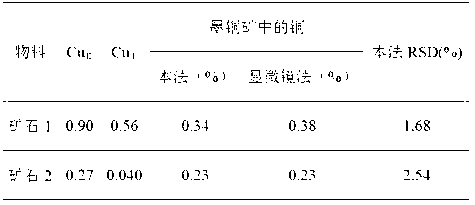
[0021] Weigh two identical samples at the same time, and perform leaching simultaneously according to the following steps respectively.
[0022] Step 1. Weigh two 0.2000g ore samples and set aside.
[0023] Step 2. Put the first ore sample in a 250mL conical flask, then add 100mL of leachate 1 to the conical flask, soak in a boiling water bath for 30 minutes, filter through slow filter paper after cooling, use 2% The washing liquid washes the precipitate and the Erlenmeyer flask 5 times, combines the washing liquid and the filtrate, heats and concentrates to 10ml, acidifies with nitric acid, adds chloride ions until no precipitation occurs, and measures after constant volume. This is the copper oxide in the sample. and secondary copper sulfide content, denoted as Cu Ⅰ .
[0024] Step 3. Put the second ore sample in a 250 mL beaker, then add 100 mL of leaching solution 3 into the beaker, and continue stirring and leaching for 3 hours. It is not suitable to use oscillation. Af...
Embodiment 2
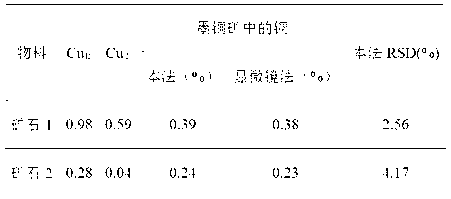
[0029] Step 1. Weigh two identical samples at the same time, and the weighing amount is 0.5000g.
[0030] Step 2. Put the first ore sample in a 250mL conical flask, then add 100mL of leachate 1 to the conical flask, soak in a boiling water bath for 50 minutes, filter through slow filter paper after cooling, and use 2% The washing liquid washes the precipitate and the Erlenmeyer flask 6 times, combines the washing liquid and the filtrate, heats and concentrates to 15ml, acidifies with nitric acid, adds chloride ions until no precipitation occurs, and measures after constant volume. This is the copper oxide in the sample. and secondary copper sulfide content, denoted as Cu Ⅰ .
[0031] Step 3. Put the second ore sample in a 250 mL beaker, then add 100 mL of leaching solution 4 into the beaker, and continue stirring and leaching for 3 hours. It is not suitable to use oscillation. After the extraction, use slow filter paper to filter. Use 1% Wash the precipitate and the beaker w...
Embodiment 3
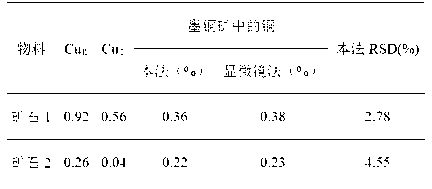
[0036] Step 1. Weigh two 0.7000g ore samples and set aside.
[0037] Step 2. Put the first mineral sample in a 250mL conical flask, then add 100mL leach solution 2 into the conical flask, leaching in a boiling water bath for 60 minutes, and filter through slow filter paper after cooling, using 2% The washing liquid washes the precipitate and the Erlenmeyer flask 6 times, combines the washing liquid and the filtrate, heats and concentrates to 20ml, acidifies with nitric acid, adds chloride ions until no precipitation occurs, and measures after constant volume. This is the copper oxide in the sample. and secondary copper sulfide content, denoted as Cu Ⅰ .
[0038] Step 3. Put the second ore sample in a 250 mL beaker, then add 100 mL of leaching solution 3 into the beaker, and continue stirring and leaching for 3 hours. It is not suitable to use oscillation. After the extraction, use slow filter paper to filter. Use 1% Wash the precipitate and the beaker with hydrochloric acid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com