Vinpocetine injection and production method thereof
A technology of vinpocetine and injection, applied in the field of pharmaceutical preparations, can solve the problems of aggravating the oxidation reaction rate, accelerating the degradation products, oxidative degradation, etc., and achieves inhibiting the degradation products of vinpocetine, ensuring sterility level, and good antioxidant performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
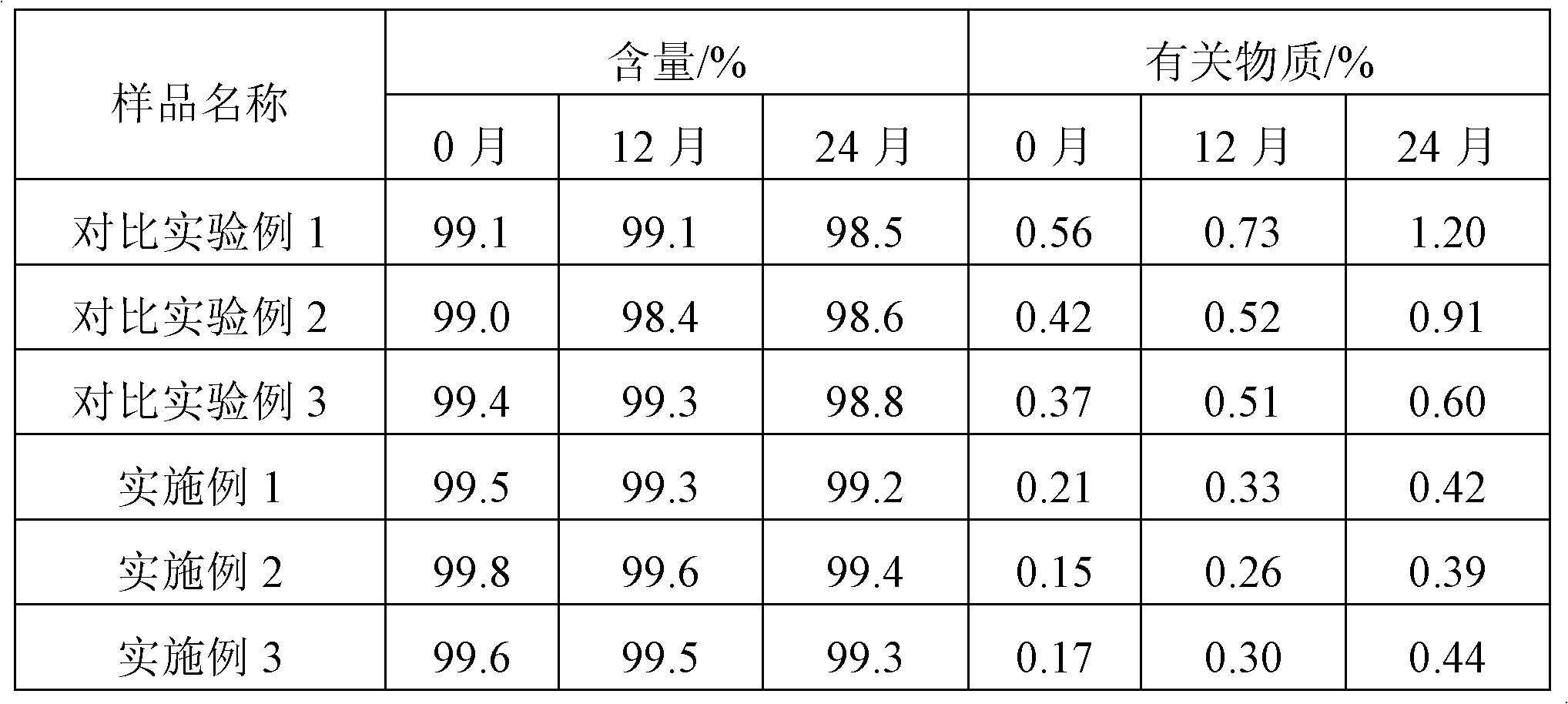
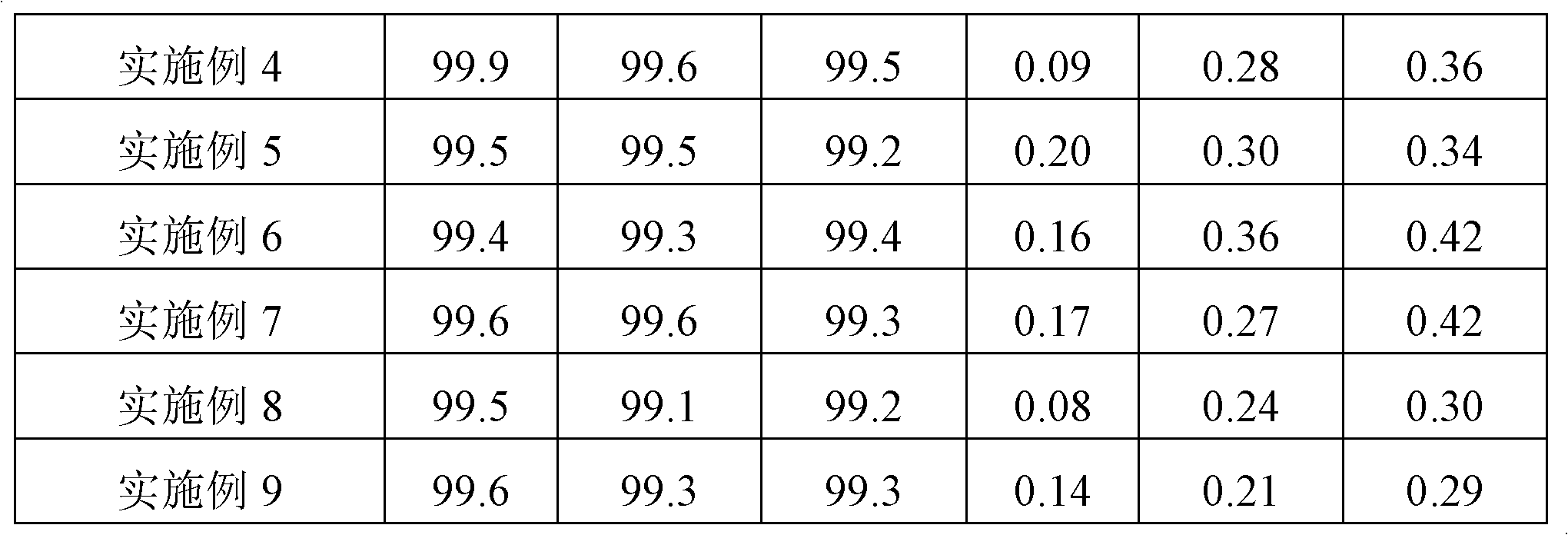
Examples
Embodiment 1
[0026] The Vinpocetine injection of the present embodiment consists of:
[0027] Vinpocetine 10g Ascorbic acid 0.3g
[0028] Sodium Metabisulfite 2g Tartaric Acid 6g
[0029] Sorbitol 160g Benzyl Alcohol 20g
[0030] Make injection 2000ml altogether.
[0031] The preparation method is as follows:
[0032] (1) 80% of the water for injection is filtered to remove oxygen, heated to 40°C, added ascorbic acid, tartaric acid and sodium metabisulfite, stirred to dissolve completely;
[0033] (2) Add Vinpocetine, stir, and dissolve completely;
[0034] (3) Add sorbitol, benzyl alcohol, stir and dissolve, make up water for injection, and use 0.3% activated carbon to stir and adsorb;
[0035] (4) Sterilize at a temperature of 121°C for 15 minutes, and fill with nitrogen.
Embodiment 2
[0037] The Vinpocetine injection of the present embodiment consists of:
[0038] Vinpocetine 12g Ascorbic Acid 0.1g
[0039] Sodium Metabisulfite 2g Tartaric Acid 8g
[0040] Sorbitol 160g Benzyl Alcohol 20g
[0041] Make injection 2000ml altogether.
[0042] The preparation method is as follows:
[0043] (1) 80% of the water for injection is filtered to remove oxygen, heated to 40°C, added ascorbic acid, tartaric acid and sodium metabisulfite, stirred to dissolve completely;
[0044] (2) Add Vinpocetine, stir, and dissolve completely;
[0045] (3) Add sorbitol, benzyl alcohol, stir and dissolve, make up water for injection, and use 0.3% activated carbon to stir and adsorb;
[0046] (4) Sterilize for 15 minutes at a temperature of 121°C, and fill with nitrogen.
Embodiment 3
[0048] The Vinpocetine injection of the present embodiment consists of:
[0049] Vinpocetine 20g Ascorbic Acid 0.45g
[0050] Sodium Metabisulfite 2g Tartaric Acid 15g
[0051] Sorbitol 160g Propylene Glycol 20g
[0052] Make injection 2000ml altogether.
[0053] The preparation method is as follows:
[0054] (1) 80% of the water for injection is filtered to remove oxygen, heated to 40°C, added ascorbic acid, tartaric acid and sodium metabisulfite, stirred to dissolve completely;
[0055] (2) Add Vinpocetine, stir, and dissolve completely;
[0056] (3) Add sorbitol, propylene glycol, stir and dissolve, make up water for injection, and use 0.3% activated carbon to stir and adsorb;
[0057] (4) Sterilize for 15 minutes at a temperature of 121°C, and fill with nitrogen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com