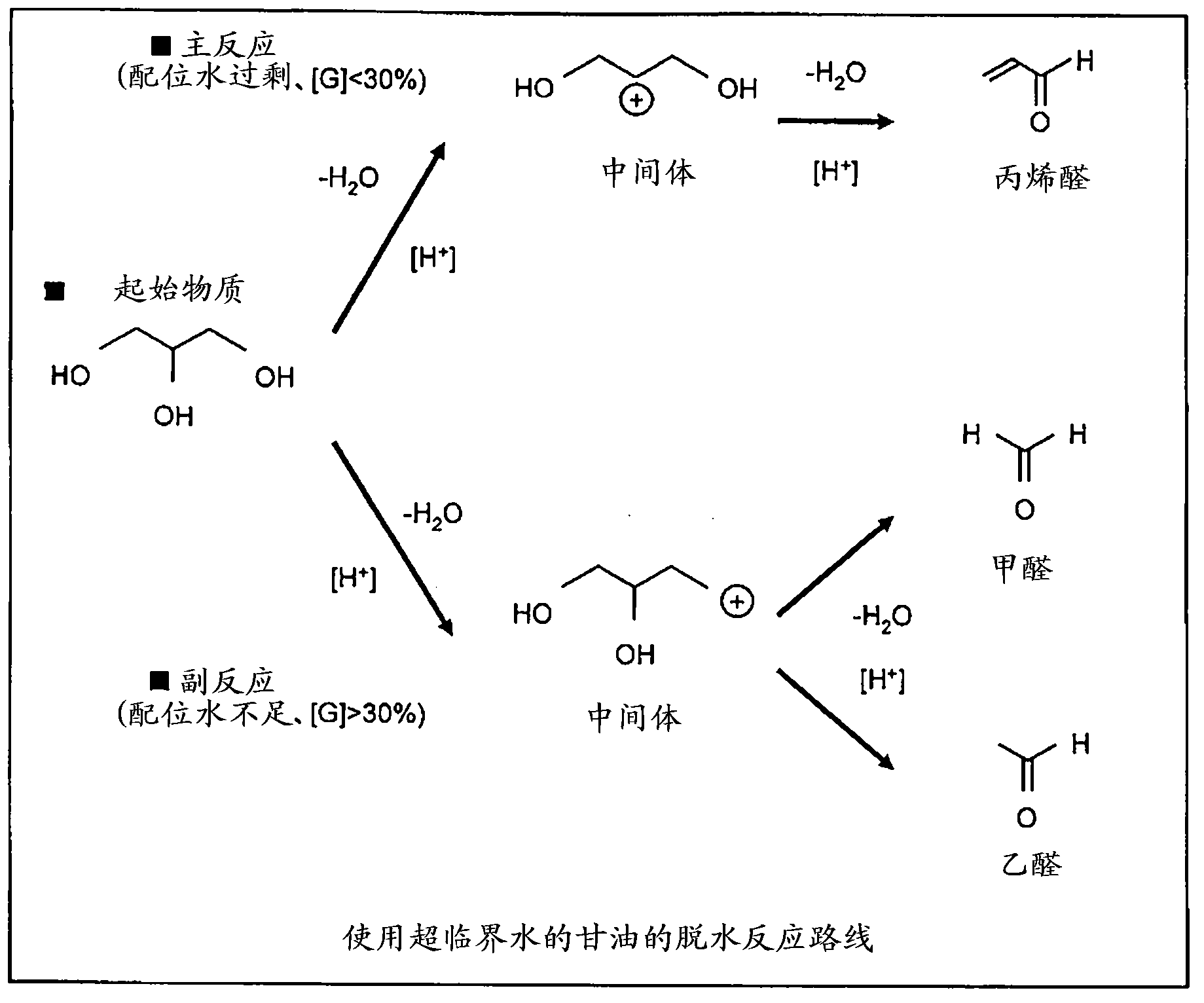
Process for synthesis of acrolein
A synthesis method and acrolein technology are applied in chemical instruments and methods, methods for chemically changing substances by using atmospheric pressure, and preparation of carbon-based compounds, which can solve problems such as reduced catalyst performance, reduced reactivity, and complicated device operation. , to reduce the amount of production, prevent pipeline blockage, and prevent wear and tear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
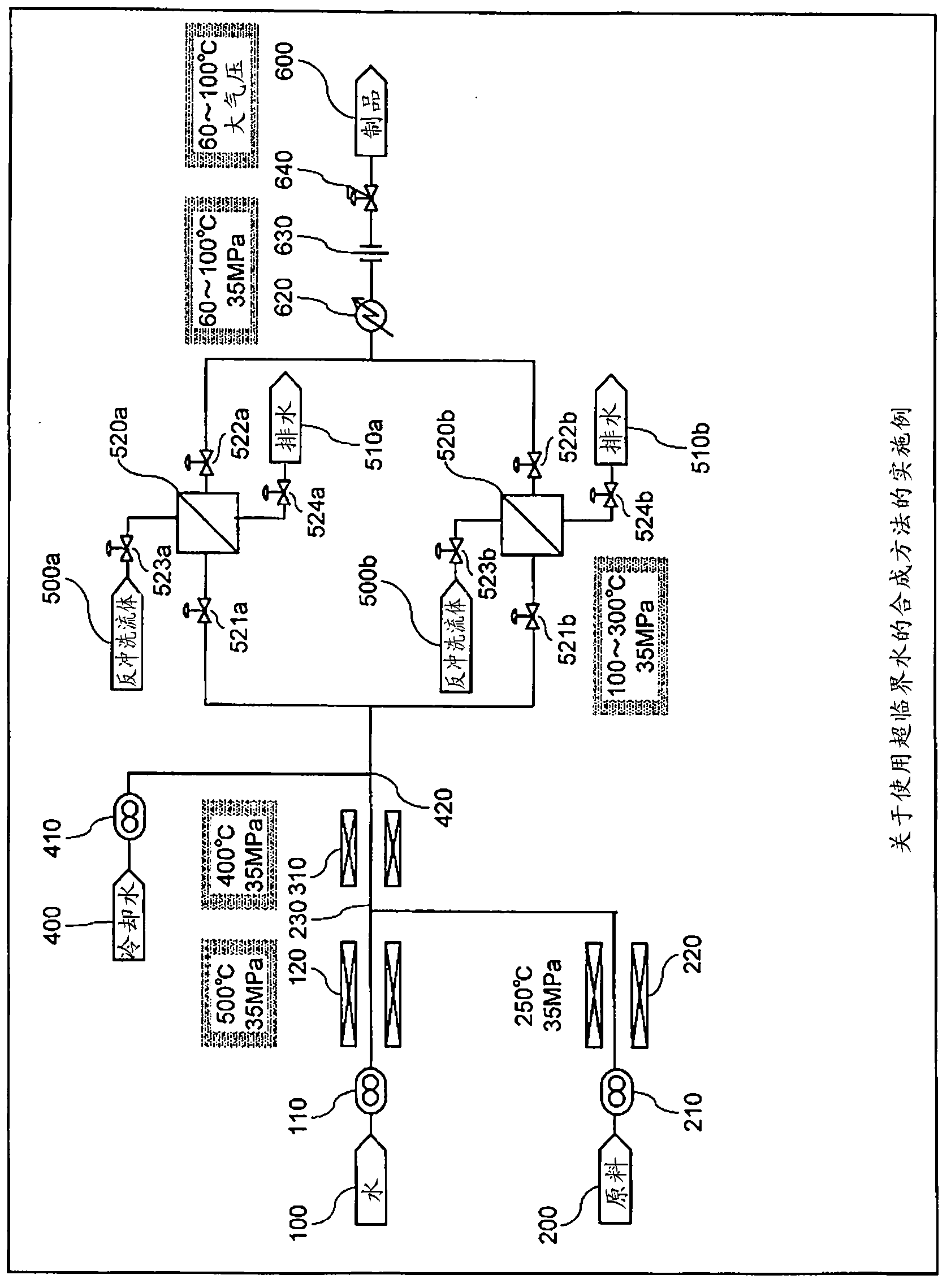
[0070] use figure 2 The supercritical reaction experimental device shown is used for acrolein synthesis experiment. The experimental conditions are shown in Table 1 (Conditions of the Optimum Reaction Condition Evaluation Experiment).
[0071] Table 1
[0072] Optimal reaction condition evaluation experiment conditions
[0073]
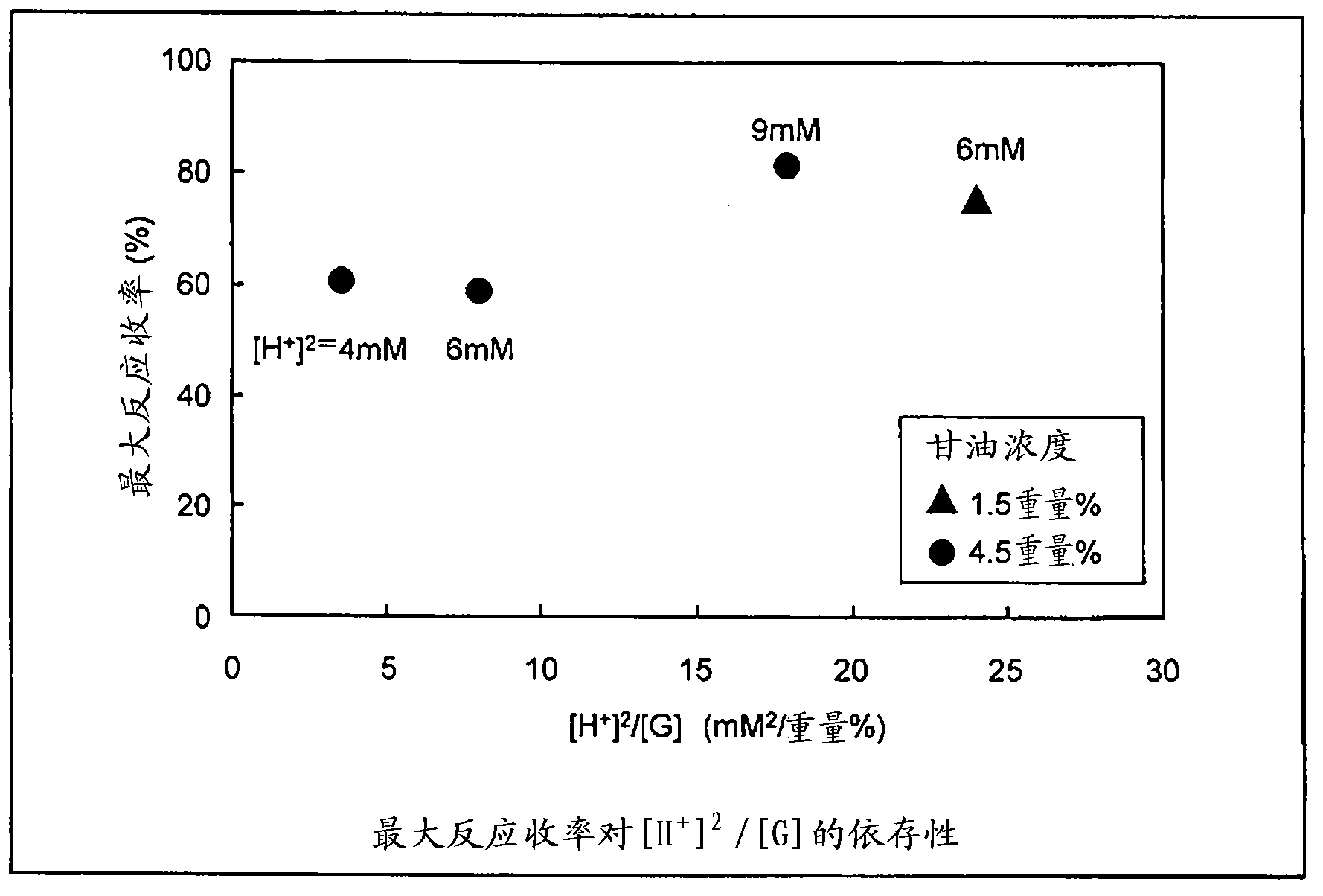
[0074] In cases A-1 to A-5, the concentration of sulfuric acid was fixed at 6 mM, and the reaction experiment was performed with the concentration of glycerol as a parameter. In addition, in cases B-1 to B-5, based on [Formula 1] and [Formula 2], reaction experiments were performed by optimizing the concentration of sulfuric acid and the reaction time according to the concentration of glycerin. The reaction yields obtained in this experiment are shown in Figure 8 . In Case A in which the test conditions were not optimized, the reaction yield was low except for Case A-1 in which the concentration of glycerin was as low as 1.5% by weight. On ...
Embodiment 2
[0077] The reaction experiment was carried out under the condition of a glycerin concentration of 30% by weight, and the separation and removal experiment of by-products was performed. The experimental conditions are shown in Table 2 (Conditions of by-product removal experiment).
[0078] Table 2
[0079] Conditions for by-product removal experiments
[0080]
[0081] The filtration temperature was set at 10°C, 200°C, and 250°C, and the filter pore diameter was set at 15 μm, 40 μm, and 60 μm. In this test, the operating time when the differential pressure of the filter reaches 6 MPa as the withstand pressure is shown in Figure 9 . In the case of a filter with a pore size of 60 μm, clogging occurred in the pressure reducing valve before the differential pressure of the filter reached the withstand pressure, so no data is recorded in the figure. On the other hand, in the case of the filter with a pore size of 15 and 40 μm, the pressure reducing valve was not clogged, and...
Embodiment 3
[0083] Figure 11 The following process is shown: acrolein is synthesized from glycerin using the supercritical water reaction process described in Examples 1 and 2 of the present invention, and further converted into 1,3-propanediol by performing hydration reaction and hydrogenation reaction. Polycondensation with terephthalic acid to obtain polytrimethylene terephthalate (PTT). When the raw material glycerin contains impurities such as organic acids (hydrolysates of oils and fats used as raw materials for biodiesel fuels, etc.) or metal salts (alkali catalysts or their reactants used to prepare biodiesel fuels), it is preferable to use separation and / or These are removed by filtration, ion exchange, distillation, etc. This is because the presence of organic acids is the reason for the low yield of raw materials. Even if salts are soluble in water at normal temperature and pressure, they may be precipitated in supercritical water with a small dielectric constant and adhere t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| aperture size | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| aperture size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com