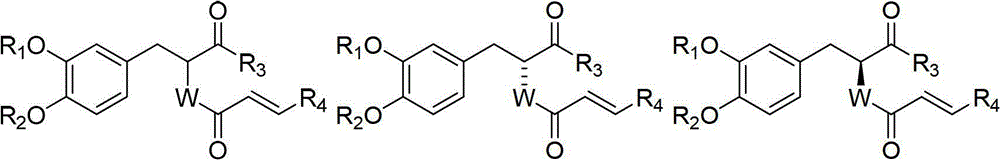
Rosmarinic acid derivative, its preparation method, and its application in preparation of antitubercular medicines
A technology of rosmarinic acid and its derivatives, which can be used in antibacterial drugs, organic chemistry, etc., and can solve the problem of blood drug concentration reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
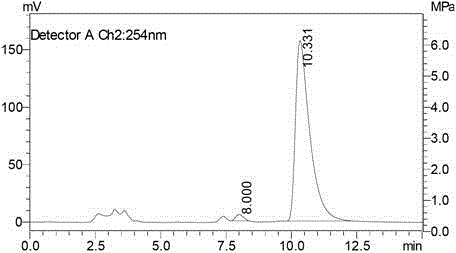
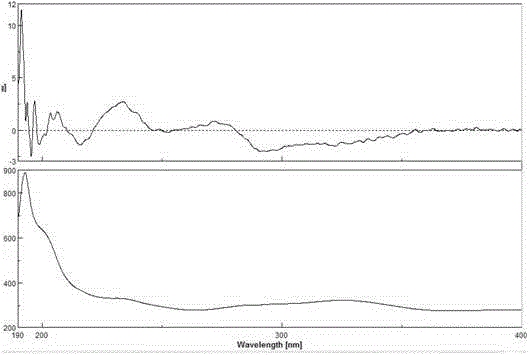
Image
Examples
Embodiment 1
[0058] Embodiment 1, the synthesis of (Z)-4-(3,4-dimethoxymethylenebenzene)-2-methyloxazolone
[0059]
[0060]Add veratraldehyde (10mmol, 1.66g), acetylglycine (10mmol, 1.17g), anhydrous sodium acetate (20mmol, 1.64g) and 20mL anhydrous acetic anhydride in a 50mL round bottom flask, stir to make it dissolve, and heat up to After reacting at 110°C for 8 hours, cool to room temperature, add 20 mL of ice water, crush the solid in the bottle, filter, wash the filter cake (5×10 mL) with distilled water, and then wash with 50% ethanol (5×10 mL), after drying, Recrystallized with acetone to obtain 2.11 g of yellow crystalline product (Z)-4-(3,4-dimethoxymethylenebenzene)-2-methyloxazolone with a yield of 85%; m.p.166~168°C ; 1 H NMR (DMSO-d 6 , 300MHz): δ2.37(s, 3H, CH 3 ), 3.83 (s, 3H, OCH 3 ), 3.86(s, 3H, OCH 3 ), 7.01(d, J=7.7Hz, 1H, ArH), 7.10(s, 1H, ArH), 7.66(d, J=7.7Hz, 1H, ArH), 7.93(s, 1H, CH); ESI- MS m / z 270.1[M+Na] + , 246.1[M-H] - ;HRMS(ESI):m / z calcd for C ...
Embodiment 2
[0061] The synthesis of embodiment 2,3,4-dimethoxyphenylpyruvate
[0062]
[0063] Add (Z)-4-(3,4-dimethoxymethylenebenzene)-2-methyloxazolone (9mmol, 2.01g) (2), 3M HCl 50mL, 90 Reaction at °C for 10 h. After stopping the reaction, cool to room temperature, filter, wash with water (3×10mL), dry, and wash the crude product with MeOH / H 2 O was recrystallized to obtain 1.80 g of light yellow solid 3,4-dimethoxyphenylpyruvate, with a yield of 89%; m.p.200~203°C; 1 H NMR (DMSO-d 6 , 300MHz): δ3.74(s, 3H, OCH 3 ), 3.75(s, 3H, OCH 3 ), 6.37(s, 1H, CH), 6.94(d, J=8.7Hz, 1H, ArH), 7.30(dd, J=2.1, 1.8Hz, 1H, ArH), 7.42(d, J=1.8Hz, 1H, ArH), 8.99 (brs, 1H, OH), 13.02 (brs, 1H, COOH); ESI-MS m / z 247.0 [M+Na] + , 471.0[2M+Na] + , 222.9[M-H] - ; HRMS (ESI): m / z calcd for C 11 h 12 o 5 Na[M+Na] + : 247.0582, found 247.0598.
Embodiment 3
[0064] The synthesis of embodiment 3,3,4-dimethoxyphenylalanine
[0065]
[0066] Add 3,4-dimethoxyphenylpyruvate (6.7mmol, 1.5g) and 30mL of 25% methanol into a 50mL round-bottomed flask, stir in an ice-water bath until the solid dissolves, adjust the pH to 10 with 10% NaOH solution, Add NaBH in batches 4 (10mmol, 0.54g), after the addition, react at room temperature overnight. 0.1 M HCl to make acidic, extracted with ethyl acetate (4 × 25mL), combined organic phase, anhydrous MgSO 4 Dry, filter, evaporate to dryness, and column chromatography (V (dichloromethane): V (methanol): V (acetic acid) = 90: 9: 1) to obtain light yellow solid 3,4-dimethoxyphenylalanine 1.18g, yield 78%; m.p.124~126℃; 1 H NMR (DMSO-d 6 , 300MHz) δ2.94 (dd, J=14.1, 6.9Hz, 1H, CH 2 ), 3.14 (dd, J=14.1, 14.1Hz, 1H, CH 2 ), 3.85(s, 6H, OCH 3 ), 4.48(dd, J=6.9, 4.2Hz, 1H, CH), 6.82(s, 3H, ArH); ESI-MS m / z 249.1[M+Na] + , 225.1[M-H] - ; HRMS (ESI): m / z calcd for C 11 h 14 o 5 Na[M+Na] + : 24...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com