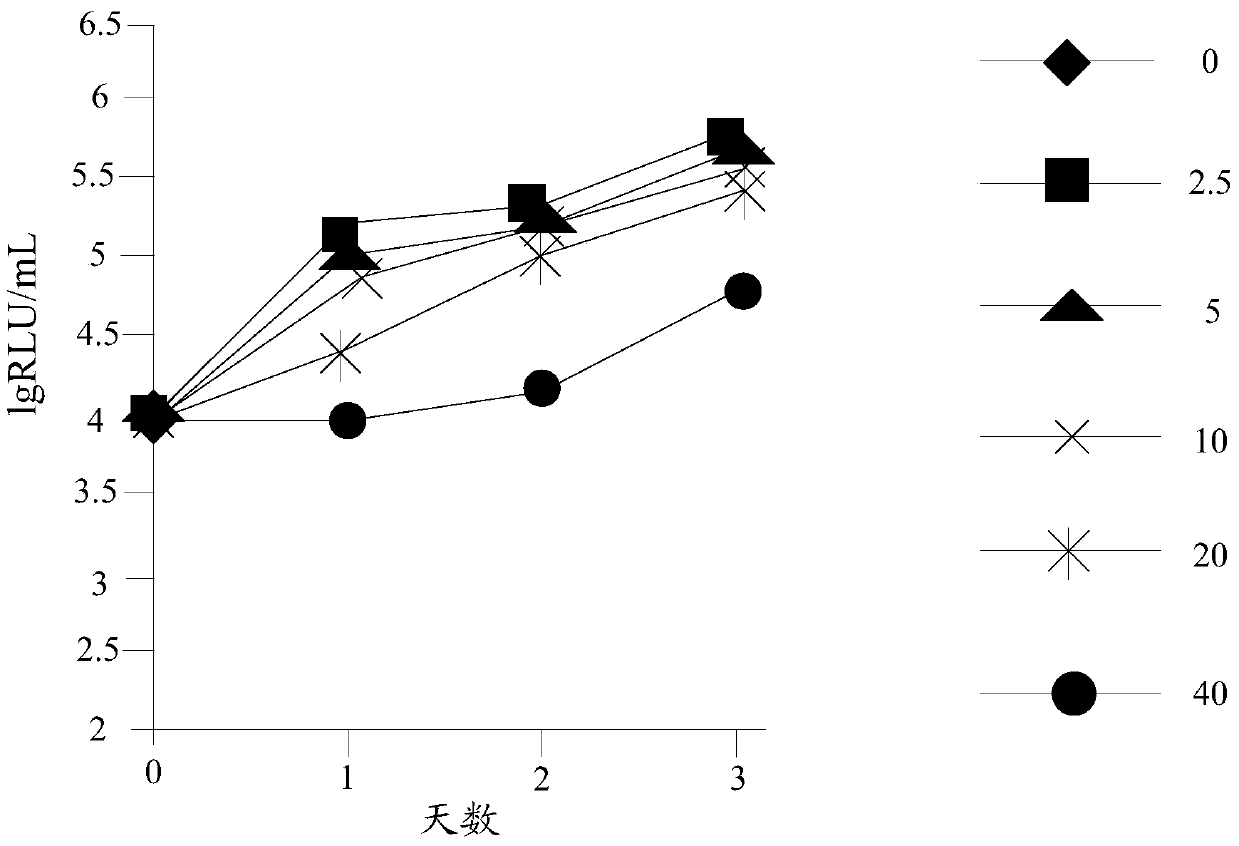
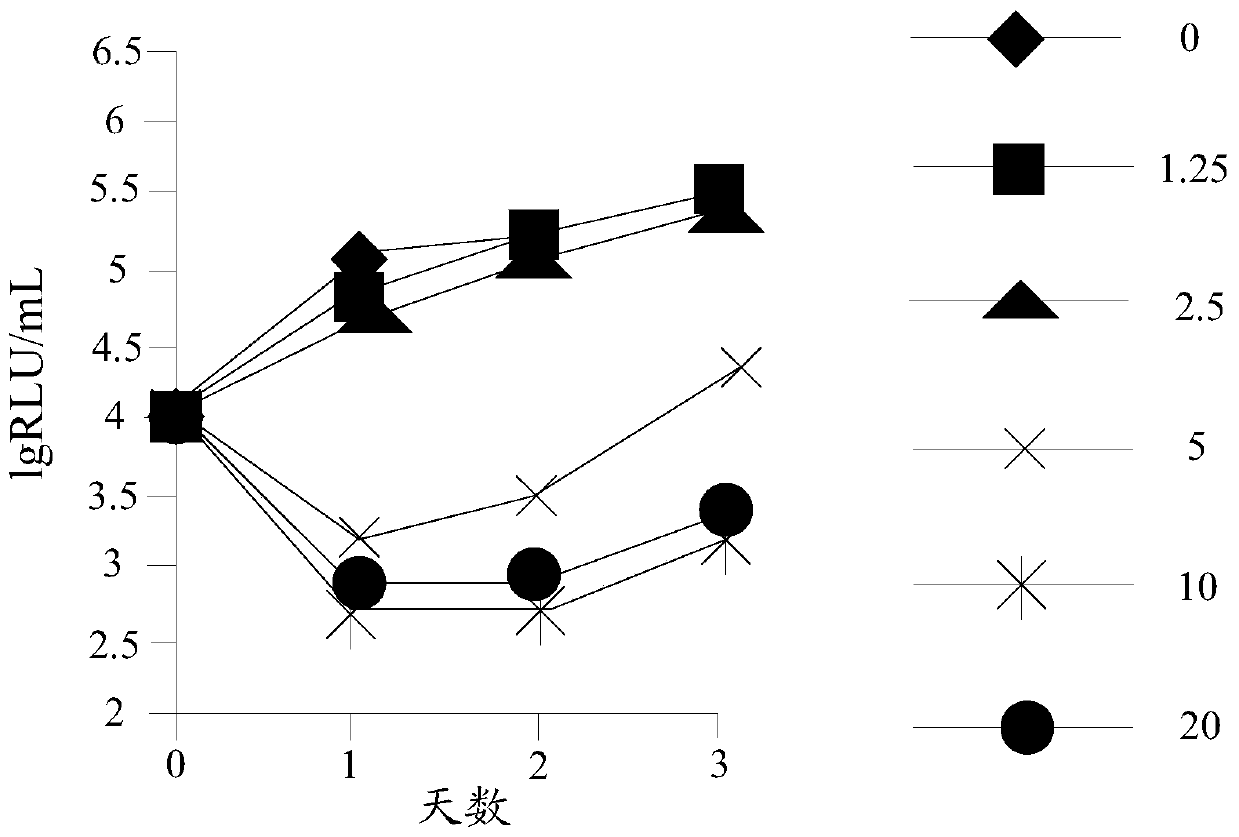
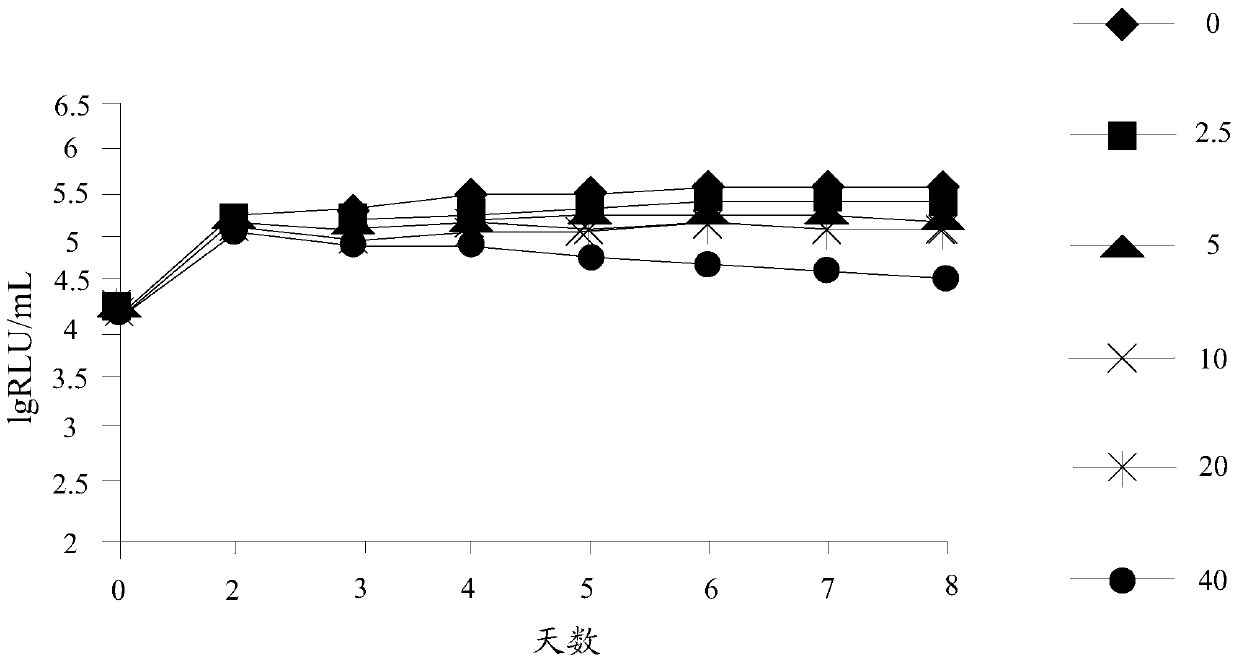
Preparation method and application of n-4-trifluoromethylphenyl salicylamide derivative
A technology of trifluoromethylphenyl salicylamide and N-4-, applied in the field of preparation of N-4-trifluoromethylphenyl salicylamide derivatives, can solve the problems of poor oral bioavailability, etc. Achieve good anti-tuberculosis activity, good anti-tuberculosis activity, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The structural formula of compound 11 is:
[0047]
[0048] The chemical reaction equation for the preparation of compound 11 is:
[0049]
[0050]The preparation method of compound 11 comprises the following steps:
[0051] 2-Hydroxy-5-methoxyformylbenzoic acid (0.2g, 1.0mmol) and p-trifluoromethylaniline (0.15mL, 1.2mmol) were dissolved in xylene and heated to 110°C under argon atmosphere Add PCl dropwise 3 (0.09mL, 1.0mmol), at this time the system changed from colorless and clear to yellow turbid state. React at 110°C for 3 hours, wait for the reaction system to cool down to room temperature, add a large amount of ethyl acrylate (EA) to dissolve, wash the organic phase with saturated saline and water, collect the organic phase, dry and concentrate, column chromatography gives light yellow Compound 11, the mass is 0.14g, and the yield is 41.2%.
[0052] The proton nuclear magnetic resonance spectrum data of compound 11 is:
[0053] 1 H-NMR: (400MHz, d-CHC...
Embodiment 2
[0055] The structural formula of compound 12 is:
[0056]
[0057] The chemical reaction equation for the preparation of compound 12 is:
[0058]
[0059] The preparation method of compound 12 is:
[0060] 2-Hydroxy-5-acetylbenzoic acid (1.0mmol) and p-trifluoromethylaniline (0.15mL, 1.2mmol) were dissolved in xylene, heated to 130°C, and PCl was added dropwise under argon atmosphere 3 (0.09mL, 1.0mmol), at this time the system changed from colorless and clear to yellow turbid state. React at 130°C for 3 hours, wait for the reaction system to cool to room temperature, add a large amount of ethyl acrylate (EA) to dissolve, wash the organic phase with saturated saline and water, collect the organic phase, dry and concentrate, column chromatography gives light yellow The solid, that is, compound 12, has a mass of 0.14 g and a yield of 41.2%.
[0061] The proton nuclear magnetic resonance spectrum data of compound 12 is:
[0062] 1 H-NMR: (400MHz, d-CHCl 3 )12.48(s, 1H...
Embodiment 3
[0064] The structural formula of compound 13 is:
[0065]
[0066] The chemical reaction equation for the preparation of compound 13 is:
[0067]
[0068] The preparation method of compound 13 is:
[0069] 2-Hydroxy-5-nitrobenzoic acid (1.0mmol) and p-trifluoromethylaniline (0.15mL, 1.2mmol) were dissolved in xylene and heated to 120°C, and PCl was added dropwise in an argon atmosphere 3 (0.09mL, 1.0mmol), at this time the system changed from colorless and clear to yellow turbid state. React at 120°C for 3 hours, wait for the reaction system to cool to room temperature, add a large amount of ethyl acrylate (EA) to dissolve, wash the organic phase with saturated saline and water, collect the organic phase, dry and concentrate, column chromatography gives light yellow The solid, namely compound 13, yielded 66.2%.
[0070] The proton nuclear magnetic resonance spectrum data of compound 13 is:
[0071] 1 H-NMR: (400MHz, d-DMSO) 10.83(s, 1H), 8.68(s, 1H), 8.29(d, J=9.2Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com