anti-tuberculosis drugs
A drug and technology for tuberculosis, applied in the field of anti-tuberculosis drugs, can solve the problems of low ALR inhibitory activity, no obvious improvement of toxicity in DCS structure modification, toxic and side effects of human body, etc., and achieve good anti-tuberculosis activity, low cytotoxicity, and anti-tuberculosis bacteria. Broad spectrum effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
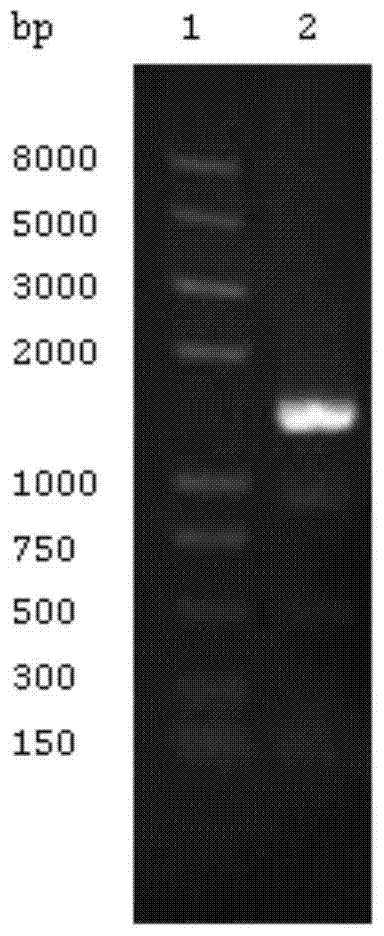
[0048] Example 1 Construction of expression vector for MTB ALR
[0049] Using MTB H37Rv genome as template, with ALR-PF: 5’CGG GAATTC ATGAAACGGTTCTGGGAGAATGTCGGAAAGC3’ (SEQ ID NO: 1) and ALR-PR: 5’CAT AAGCTT ACCACGGTTTTCAGCCTCGCGATAGGTCCT3' (SEQ ID NO: 2) is the primer (underline is the restriction site), PCR amplification is carried out, and the conditions are: 95°C pre-denaturation for 5min; 98°C denaturation for 20s, 70°C renaturation for 15s, 72°C for extension , 30 cycles; the final reaction at 72°C for 8 min. The PCR product was digested with EcoR I and Hind III and then ligated with the expression vector pET28a and transformed into E.coli DH5α. Screen positive clones on the LB plate containing 50mg / L Kanamycin (Kan), transform the sequenced plasmid into E.coli BL21(DE3)pLysS, and select the stable ALR protein on the LB plate containing 50mg / L Kan Expressed strain. For basic operations, please refer to the Molecular Cloning Experiment Guide [12] .
[0050] Result: The PC...
Embodiment 2
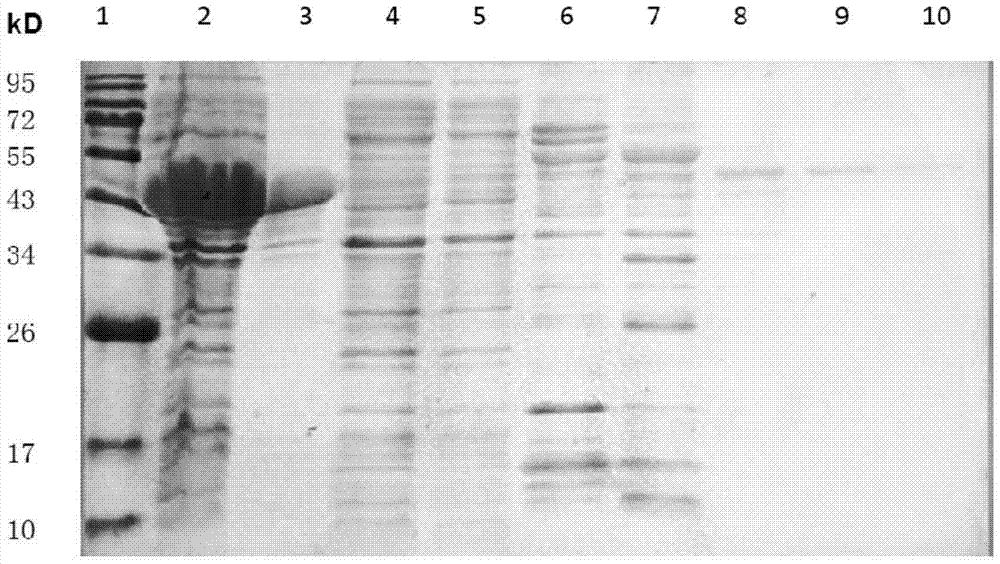
[0051] Example 2 Expression and purification of MTB ALR
[0052] Pick a single colony in LB liquid medium, culture it overnight at 37°C and 200r / min, inoculate it in LB medium containing 100mmol / L sorbitol at a ratio of 1:100, and cultivate to OD 600 = 0.6. Add IPTG to a final concentration of 20μmol / L, add lactose to a final concentration of 5mmol / L, and induce overnight at 16°C and 200r / min. Collect bacteria by centrifugation at low temperature, add lysis buffer [50mmol / L NaH 2 PO 4 , 300mmol / L NaCl, 30mmol / L imidazole, pH 8.0], liquid nitrogen-ice water repeated freezing and thawing 5 times, and then using a cell ultrasonic disruptor at 70% energy, 3s / 8s, 100 cycles of disruption, 8000× Collect the supernatant after centrifugation at g for 10 min. Use Ni + Affinity chromatography column separates the target protein, add PLP to a final concentration of 0.5mmol / L before loading, and use elution buffer [50mmol / L NaH 2 PO 4 , 300mmol / L NaCl, 500mmol / L imidazole, pH 8.0] and lysis...
Embodiment 3
[0054] Example 3 Enzyme activity determination and optimization of screening conditions
[0055] The enzyme activity reaction was carried out in a 96-well microtiter plate with a final volume of 100μl. Enzyme activity determination system includes: 100mmol / LTris-HCl (pH8.0), 10mmol / L NAD, 0.1U / mL alanine dehydrogenase (Alanine dehydrogenase, ALD), 10mmol / L D-alanine (D-alanine) Acid), 0.3μg ALR. Detect the absorbance value (OD) of the reaction system at 340nm within 40min at 37℃ 340 )Variety. Among them, DMSO is the solvent and negative control of the compound. Enzyme stability was measured as the ALR activity after treatment at temperatures of 4, 25, 30, 34, 37, 42, 48, 52, 58, and 65°C for 30 min. The optimized parameters of the screening conditions include: reaction temperature (28, 31, 34, 37, 40, 45°C), reaction pH (2-11, 17 pH values are selected at intervals), reaction substrate NAD (20~0.1625mmol / L, double dilution) and D-alanine (20~0.1625mmol / L, double dilution),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com