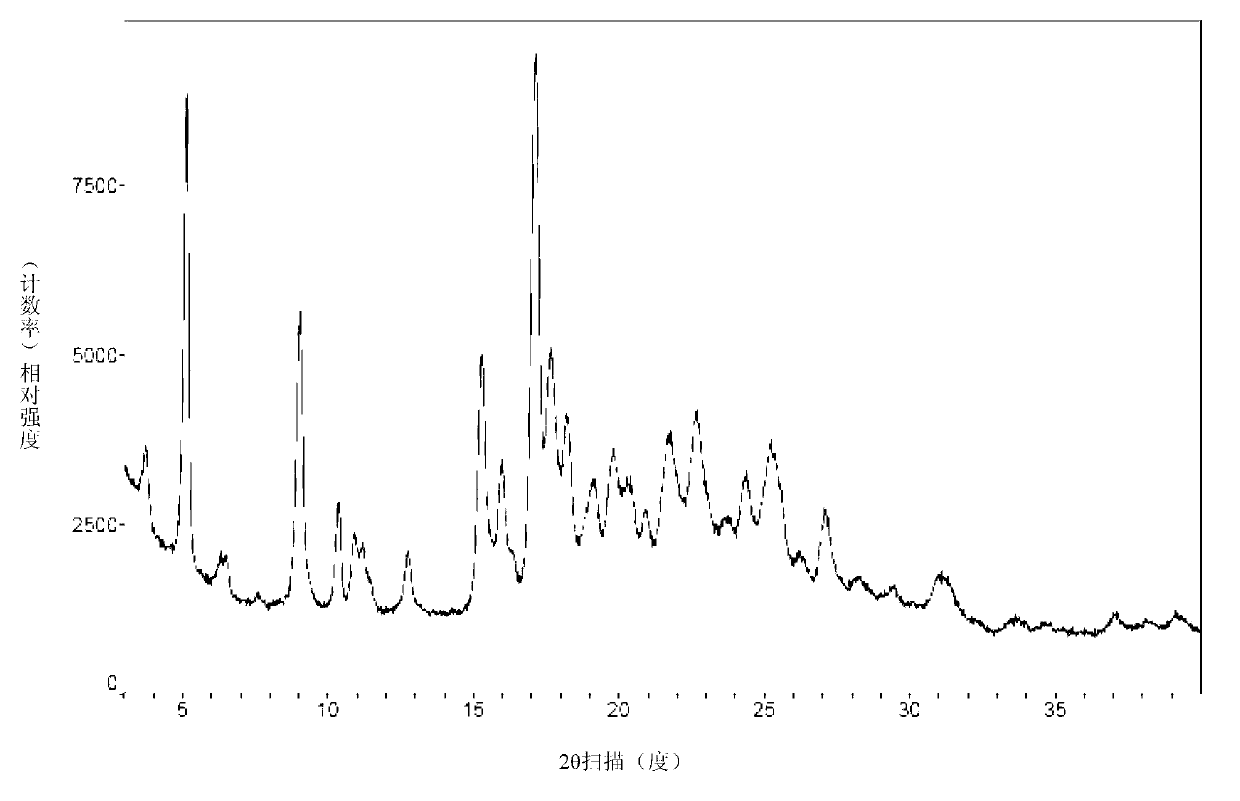
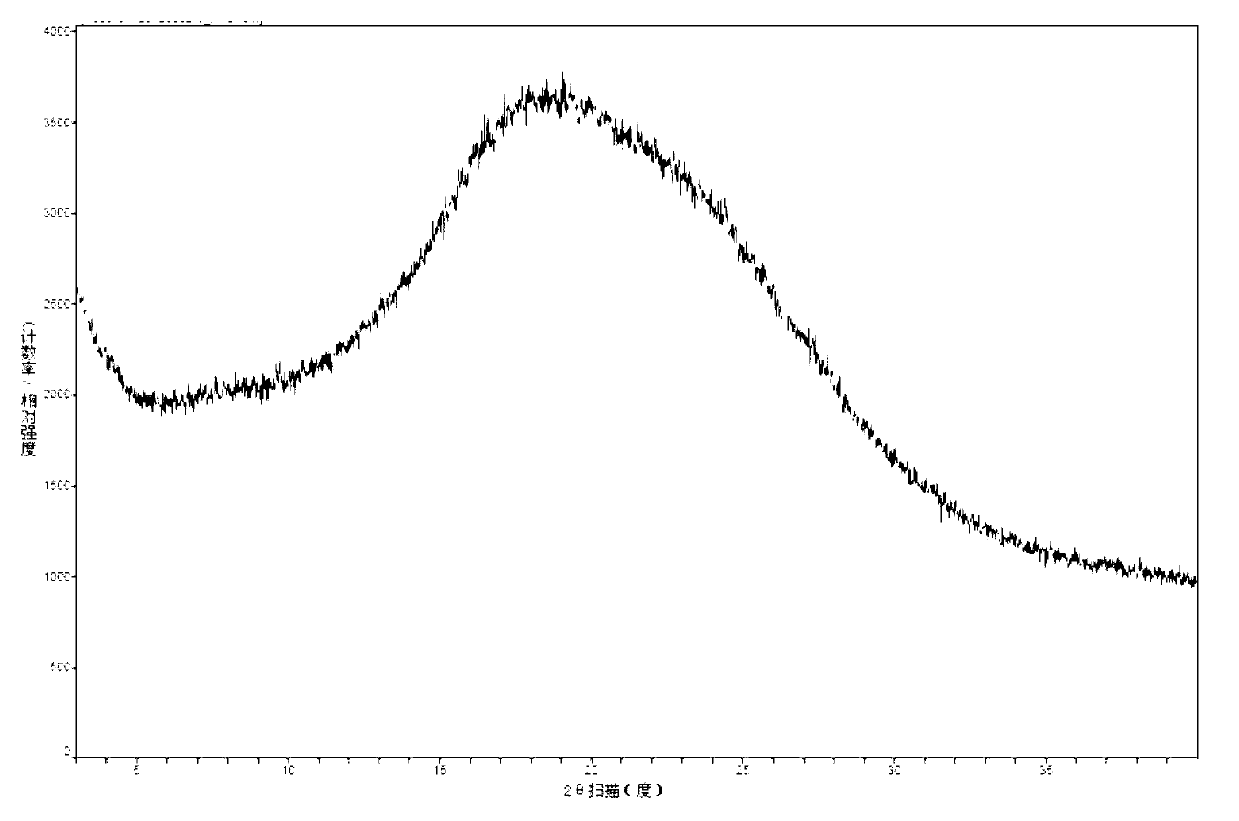
Crystal ertapenem intermediate as well as preparation method and application thereof
A crystallization and crystallization technology, applied in the field of ertapenem intermediates and its preparation, to achieve the effects of improved purity, good separability, and shortened filtration time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Embodiment 1: the preparation of formula I compound crystal
[0088] (1R,5R,6S)-6-[(1R)-1-Glycolic acid]-2-[(Diphenoxyphosphoryl)oxy]-1-methylcarbapenicill-2-ene-3 - p-nitrobenzyl formate (carbapenem nucleus, compound of formula III) (36.0g, 0.0605mol) was dissolved in 300ml DMF, and the side chain of ertapenem (compound of formula IV) (26.7g, 0.0599 mol) diisopropylamine (DIPA, 9.4 g, 0.0727 mol) was added dropwise at -35°C and stirred for reaction. After the reaction is completed, add it to aqueous hydrochloric acid solution (pH=4), and filter to obtain an amorphous white or off-white solid. Then add the solid to 320ml of ethyl acetate, stir and dissolve at 0°C, wash twice with 80ml of 5% (w / w) potassium dihydrogen phosphate aqueous solution each time, separate the organic phase, and then wash it with 10g of anhydrous sodium sulfate Dry, filter with suction, and stir the filtrate (solution concentration about 0.15g / ml) at 0°C (stirring speed 80 rpm) for 5h. The p...
Embodiment 2
[0093] Embodiment 2: the preparation of formula I compound crystal
[0094] (1R,5R,6S)-6-[(1R)-1-Glycolic acid]-2-[(Diphenoxyphosphoryl)oxy]-1-methylcarbapenicill-2-ene-3 - p-nitrobenzyl formate (compound of formula III) (36.0g, 0.0605mol) was dissolved in 300ml DMF, and the side chain of ertapenem (compound of formula IV) (26.7g, 0.0599mol) was added at -35°C Diisopropylamine (9.4 g, 0.0727 mol) was added dropwise and the reaction was stirred. After the reaction is completed, add it to aqueous hydrochloric acid solution (pH=4), and filter to obtain an amorphous white or off-white solid. Then add the solid to 365ml of ethyl acetate, stir and dissolve at 5°C, wash twice with 80ml of 5% (w / w) potassium dihydrogen phosphate aqueous solution each time, separate the organic phase, and then dry it with 10g of anhydrous sodium sulfate , filtered with suction, and the filtrate (solution concentration about 0.13 g / ml) was added with 0.5 g of the crystalline compound obtained in Exa...
Embodiment 3
[0099] Embodiment 3: the preparation of formula I compound crystal
[0100](1R,5R,6S)-6-[(1R)-1-Glycolic acid]-2-[(Diphenoxyphosphoryl)oxy]-1-methylcarbapenicill-2-ene-3 - p-nitrobenzyl formate (compound of formula III) (36.0g, 0.0605mol) was dissolved in 300ml DMF, and the side chain of ertapenem (compound of formula IV) (26.7g, 0.0599mol) was added at -35°C Diisopropylamine (9.4 g, 0.0727 mol) was added dropwise and the reaction was stirred. After the reaction is completed, add it to aqueous hydrochloric acid solution (pH=4), and filter to obtain an amorphous white or off-white solid. Then add the solid to 320ml of isopropyl acetate and stir to dissolve at 10°C, wash twice with 80ml of 5% (w / w) potassium dihydrogen phosphate aqueous solution each time, separate the organic phase, and dry it with 10g of anhydrous sodium sulfate , suction filtration, and the filtrate (solution concentration about 0.15g / ml) was stirred at -10°C (stirring speed 100 rpm) for 3h. The product ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com