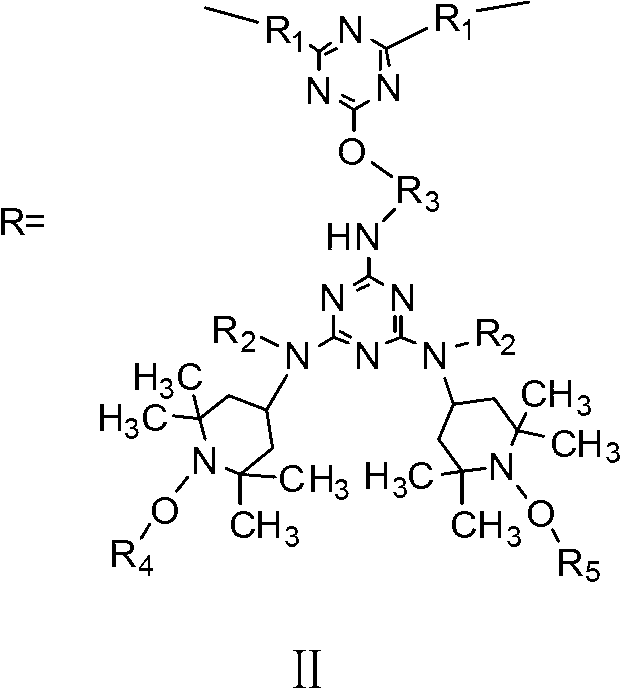
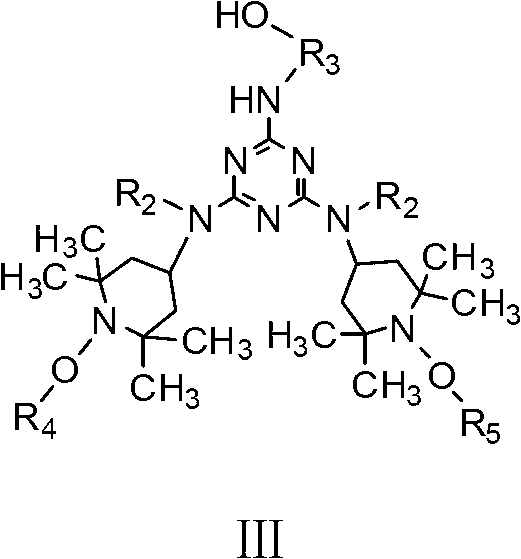
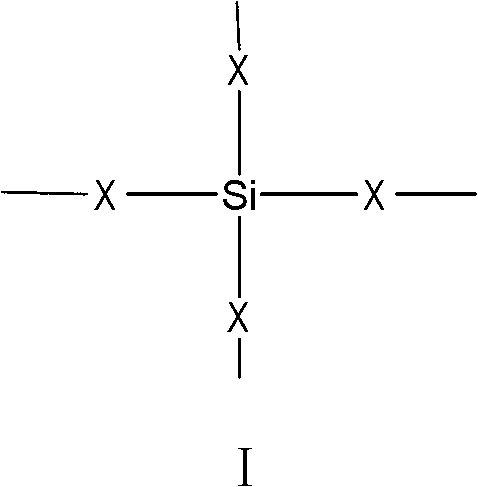Siliceous N-substituted alkyloxy hindered amine compound and its preparation method
A technology for alkoxy hindered amines and compounds, which is applied in the field of silicon-containing N-substituted alkoxy hindered amine compounds and their synthesis, can solve problems such as complex synthesis process, achieve simple operation, overcome easy migration, easy volatility and Extraction works well
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]Add 7.376g (0.04mol) of cyanuric chloride dissolved in 80ml of acetone into a 500m1 reaction kettle equipped with a thermometer and a magnet (magnetic stirrer). The temperature of the reaction system was kept at -10°C. 7.572g (0.01mol) of the first compound (wherein, R 2 for alkyl-C 2 h 5 , R 3 for alkyl-C 2 h 4 -, R 4 is alkyl-CH 4 , R 5 Cycloalkyl-C 3 h 6 ) and 8.70ml of acid-binding agent N,N-isopropylethane (0.05mol) were dissolved in 30ml of acetone, and were added dropwise into the reaction kettle with a micro-injector to control the rate of addition, and the reaction temperature was controlled at -10 At ℃, a substitution reaction of the chlorine atom on the triazine ring on the cyanuric chloride is carried out. After the dropwise addition, the reaction was continued at -10° C. for 5 h at a low temperature, and the pH of the reaction solution was 6.0 at this time. Finally, pour the solution in the reaction kettle into a 250ml three-necked flask and move...
Embodiment 2
[0056] Add 3.688g (0.02mol) of cyanuric chloride dissolved in 80ml of methyl ethyl ketone into a 500m1 reaction kettle equipped with a thermometer and a magnet (magnetic stirrer), control the temperature in a low-temperature thermostat and fully stir to make the reactants evenly dispersed And keep the temperature of the reaction system at 10°C. 7.572g (0.01mol) of the first compound (wherein, R 2 for alkyl-C 2 h 5 , R 3 for alkyl-C 2 h 4 -, R 4 is alkyl-CH 4 , R 5 Cycloalkyl-C 3 h 6 ) and 15ml of acid-binding agent NaOH aqueous solution (2.0mol / L) were dissolved in 30ml of methyl ethyl ketone, and were added drop by drop to the reaction kettle at the same time with a micro-injector to control the rate of addition, and the reaction temperature was controlled at 10°C. A substitution reaction of the chlorine atom on the triazine ring on the polycyanogen chloride. After the dropwise addition, the reaction was continued at a low temperature of 10° C. for 2 h, and the pH ...
Embodiment 3
[0068] Add 1.844g (0.01mol) of cyanuric chloride dissolved in 80ml of acetone into a 500m1 reaction kettle equipped with a thermometer and a magnet (magnetic stirrer). The temperature of the reaction system was kept at 5°C. 7.572g (0.01mol) of the first compound (wherein, R 2 for alkyl-C 2 h 5 , R 3 for alkyl-C 2 h 4 -, R 4 is alkyl-CH 4 , R 5 Cycloalkyl-C 3 h 6 ) and 0.870ml of acid-binding agent N,N-isopropylethane (0.005mol) were dissolved in 30ml of acetone, and were added dropwise into the reaction kettle with a micro-injector to control the rate of addition, and the reaction temperature was controlled at 5°C Next, carry out a substitution reaction of the chlorine atom on the triazine ring on the cyanuric chloride. After the dropwise addition, the reaction was continued at 5° C. for 3 h at a low temperature, and the pH of the reaction solution was 6.5 at this time. Finally, pour the solution in the reaction kettle into a 250ml three-necked flask and move it to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com