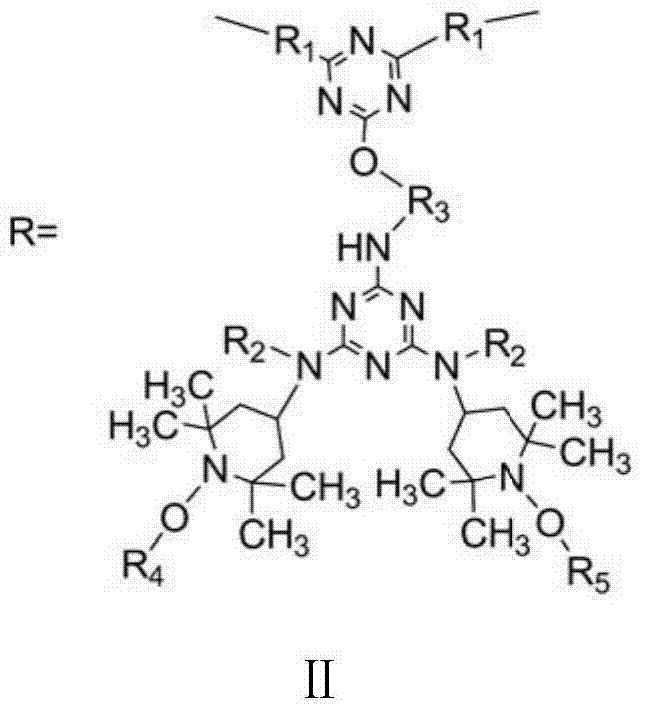
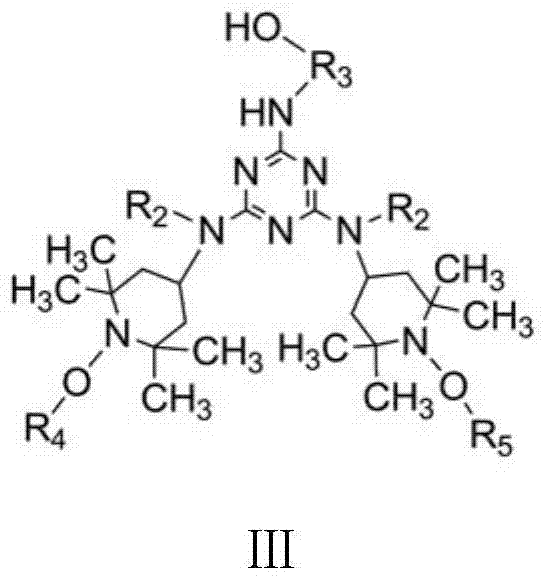
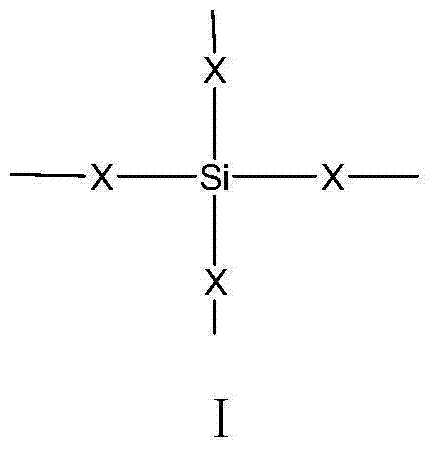Siliceous N-substituted alkyloxy hindered amine compound and its preparation method
A technology of alkoxy hindered amines and compounds, which is applied in the field of silicon-containing N-substituted alkoxy hindered amine compounds and their synthesis, can solve the problems of complex synthesis process, etc., and achieve simple operation, easy volatile extraction, energy saving and environmental protection , easy separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 7.376g (0.04mol) cyanuric chloride dissolved in 80ml acetone is added in the 500ml reaction kettle that thermometer, magneton (magnetic force stirrer) are housed, and low-temperature constant temperature bath controls temperature and fully stirs, and reactant is evenly dispersed and The temperature of the reaction system was kept at -10°C. With 7.572g (0.01mol) the first compound (wherein, R 2 for alkyl-C 2 h 5 , R 3 for alkyl-C 2 h 4 -, R 4 is alkyl-CH 3 , R 5 Cycloalkyl-C 3 h 5 ) and 8.70ml of acid-binding agent N, N-diisopropylethane (0.05mol) were dissolved in 30ml of acetone, and were added dropwise in the reactor with a microsampler to control the rate of addition, and the reaction temperature was controlled at - At 10°C, a substitution reaction of the chlorine atom on the triazine ring on the cyanuric chloride is carried out. After the dropwise addition, the reaction was continued at -10° C. for 5 h at a low temperature, and the pH of the reaction solu...
Embodiment 2
[0056] Add 3.688g (0.02mol) of cyanuric chloride dissolved in 80ml of methyl ethyl ketone into a 500ml reaction kettle equipped with a thermometer and a magnet (magnetic stirrer), and control the temperature in a low-temperature thermostat and fully stir to make the reactants evenly dispersed And keep the temperature of the reaction system at 10°C. With 7.572g (0.01mol) the first compound (wherein, R 2 for alkyl-C 2 h 5 , R 3 for alkyl-C 2 h 4 -, R 4 is alkyl-CH 3 , R 5 Cycloalkyl-C 3 h 5 ) and 15ml of acid-binding agent NaOH aqueous solution (2.0mol / L) were dissolved in 30ml of methyl ethyl ketone, and were added dropwise to the reaction kettle at the same time with a micro-injector to control the rate of addition, and the reaction temperature was controlled at 10°C. A substitution reaction of the chlorine atom on the triazine ring on the polycyanogen chloride. After the dropwise addition, the reaction was continued at a low temperature of 10° C. for 2 h, and the p...
Embodiment 3
[0068] 1.844g (0.01mol) cyanuric chloride dissolved in 80ml acetone is added in the 500ml reaction kettle that thermometer, magneton (magnetic force stirrer) are housed, and the low-temperature constant temperature bath controls temperature and fully stirs, and reactant is evenly dispersed and The temperature of the reaction system was kept at 5°C. With 7.572g (0.01mol) the first compound (wherein, R 2 for alkyl-C 2 h 5 , R 3 for alkyl-C 2 h 4 -, R 4 is alkyl-CH 3 , R 5 Cycloalkyl-C 3 h 5 ) and 0.870ml acid-binding agent N, N-diisopropylethane (0.005mol) are dissolved in 30ml acetone, drop by drop in reactor with micro-sampler, control drop rate, reaction temperature is controlled at 5 At ℃, a substitution reaction of the chlorine atom on the triazine ring on the cyanuric chloride is carried out. After the dropwise addition, the reaction was continued at 5° C. for 3 h at a low temperature, and the pH of the reaction solution was 6.5 at this time. Finally, pour the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com