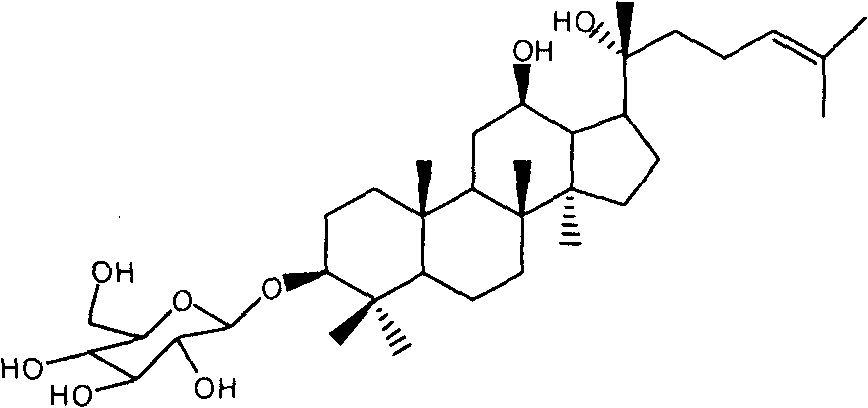
Synthetic method of 20(R)-ginsenoside Rh2
A synthetic method, ginsenoside technology, applied in the field of synthesis of rare ginsenosides, can solve problems such as difficult to increase yield, cumbersome reaction steps, large loss of raw materials, etc., and achieve low cost, simple synthetic route and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
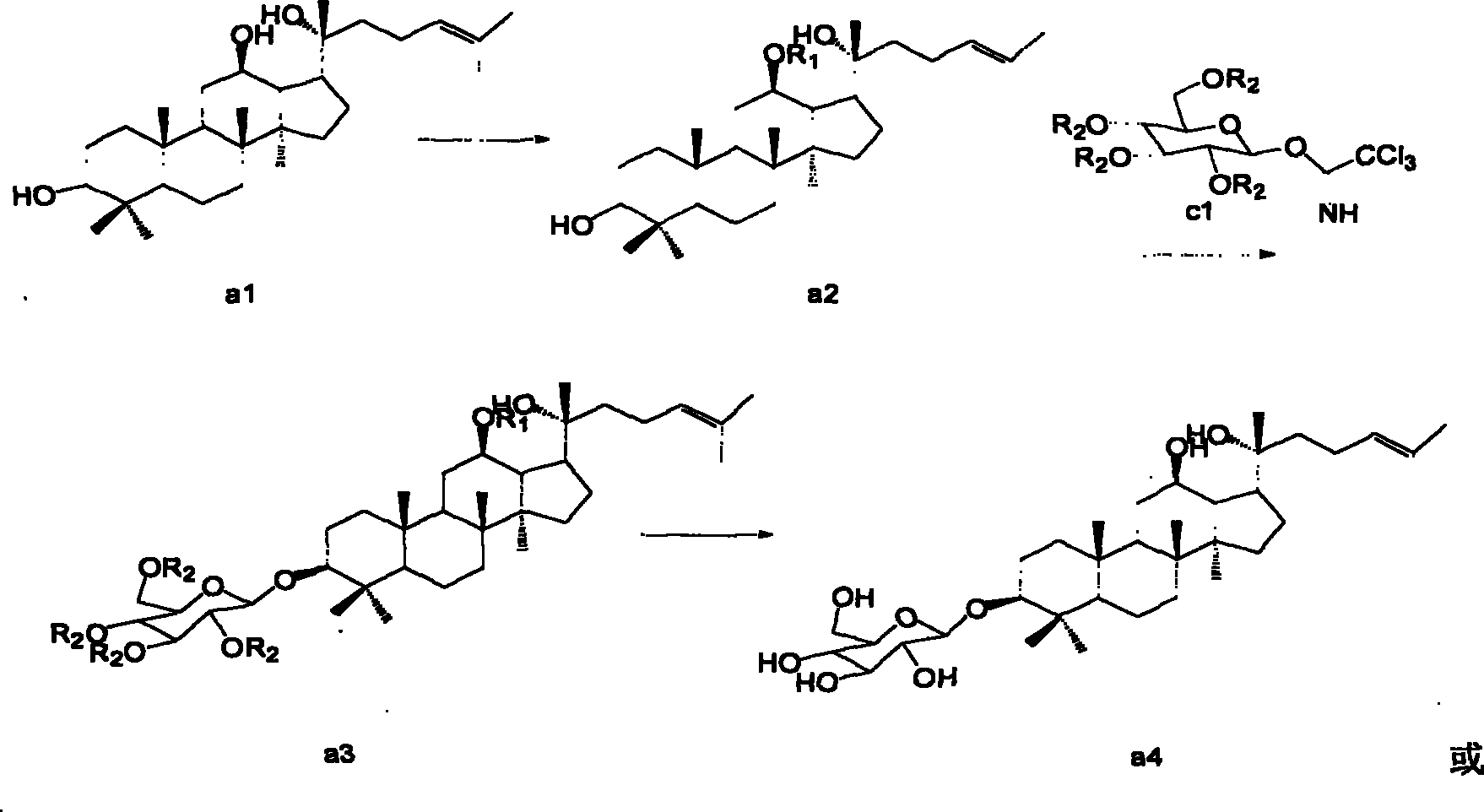
[0019] Example 1: Synthesis of 12-substituted protopanaxadiol (a2)
[0020] (1) Synthesis of 12-acetyl-20(R)-protopanaxadiol (that is, R1 is acetyl)
[0021] 20(R)-Protopanaxadiol (a1) (self-made) 4.6g (0.01mol) was dissolved in pyridine (50ml), acetic anhydride 1.5g (0.015mol) was added at room temperature, stirred overnight at 25°C, LC-MS showed After the reaction was complete, 50 ml of ice water was added to quench the reaction, concentrated under reduced pressure, the aqueous phase was extracted with ethyl acetate, washed with saturated brine until neutral, and dried over anhydrous sodium sulfate. After concentration under reduced pressure, the crude product was subjected to silica gel column chromatography (eluent: volume ratio of petroleum ether to ethyl acetate 4:1) to obtain 4.3 g of compound (a2) with a yield of 85.1% and a HPLC purity of 93.2%.
[0022] (2) Synthesis of 12-acetyl panaxadiol (that is, R1 is acetyl)
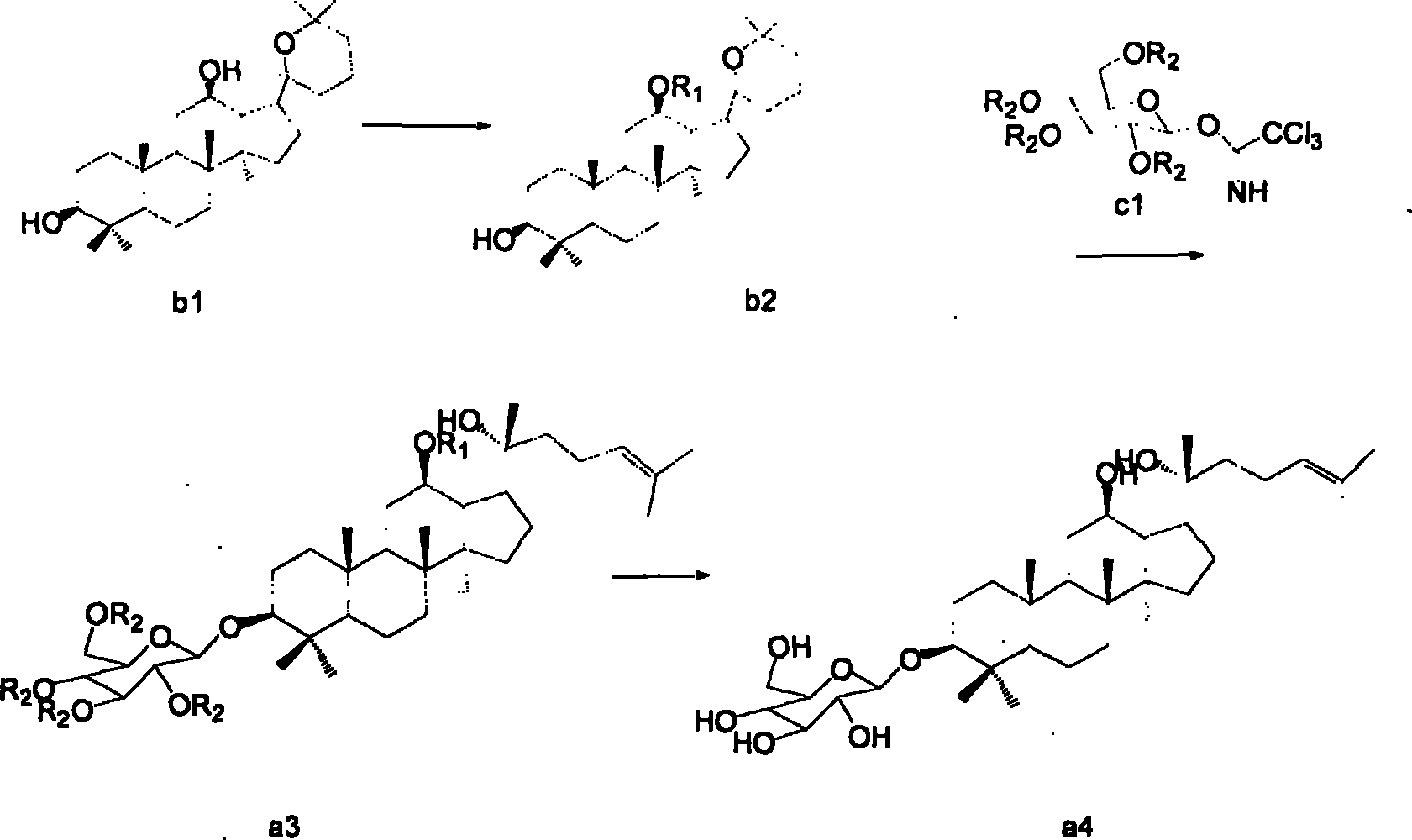
[0023] Panaxadiol (b1) (self-made) 4.6g (0.01mol)...
Embodiment 2
[0024] Embodiment 2: glycosylation reaction
[0025] (1) 500mg (0.001mol) of compound (a2) and compound c1 (prepared according to the method of Shenyang Pharmaceutical University Journal 2005 / 22 / 05, R2 is acetyl) 590mg (0.0012mol) were dissolved in 10ml of anhydrous dichloromethane Add 200 mg of 4A molecular sieves, stir at room temperature for 0.5 hours, add 100 mg of Amberlyst 15, and react with stirring at 20°C for 2 hours. After the reaction was completed, the filtrate was concentrated, and the 200-300 mesh silica gel column chromatography, eluent: the volume ratio of sherwood oil and ethyl acetate was 2: 1, to obtain 700 mg of compound (a3), with a yield of 82%, and the purity determined by HPLC was 92.13%.
[0026] (2) 500mg (0.001mol) of compound (b2) and compound c1 (prepared according to the method of Shenyang Pharmaceutical University Journal 2005 / 22 / 05, R2 is acetyl) 590mg (0.0012mol) were dissolved in 10ml of anhydrous dichloromethane , add 200 mg of 4A molecular...
Embodiment 3
[0027] Embodiment 3: deprotection group reaction
[0028] (1) Dissolve 830 mg (0.001 mol) of compound (a3) in 10 ml of methanol, add 680 mg (0.01 mol) of sodium ethoxide in batches under stirring, react at 50° C. for 8 hours, and the reaction is complete. The reaction solution was concentrated to obtain a white solid, which was recrystallized from acetonitrile to obtain 510 mg of compound (a4) with a yield of 83.89% and a purity of 99% as determined by HPLC.
[0029] (2) 830 mg (0.001 mol) of compound (b3) was dissolved in 10 ml of methanol, and 560 mg (0.01 mol) of potassium hydroxide was added in batches under stirring, and reacted at 50° C. for 8 hours, and the reaction was completed. The reaction solution was concentrated to obtain a white solid, which was recrystallized from acetonitrile to obtain 480 mg of compound (a4) with a yield of 82.01% and a purity of 99.2% as determined by HPLC.
[0030] Compound (a4) was tested by TLC, LC-MS, 1 H-NMR, 13 C-NMR, IR, 13 C- ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com