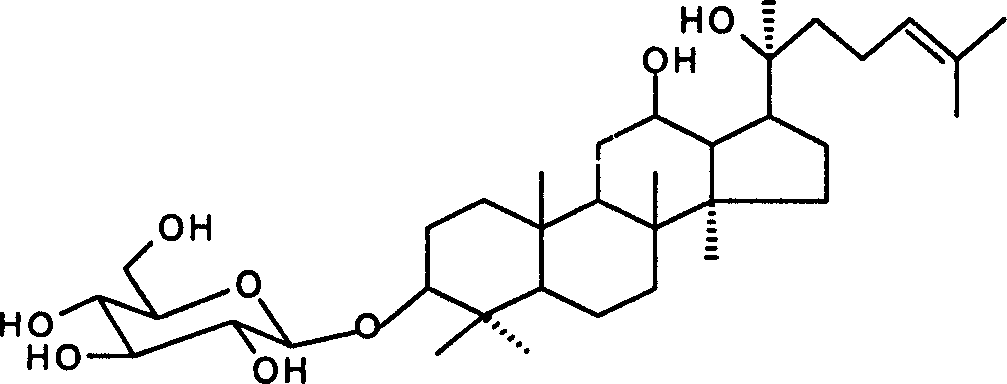
Process for synthesizing 20(S)-ginsenoside Rh2
A synthesis method and technology of ginsenosides are applied in the directions of steroids, organic chemistry, etc., can solve the problems of low yield, difficult separation, unsuitable for large-scale industrial production, etc., and achieve high selectivity, low cost, and cheap reaction raw materials. easy-to-get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
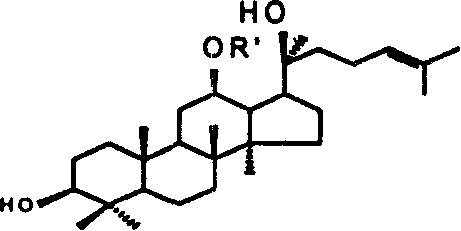
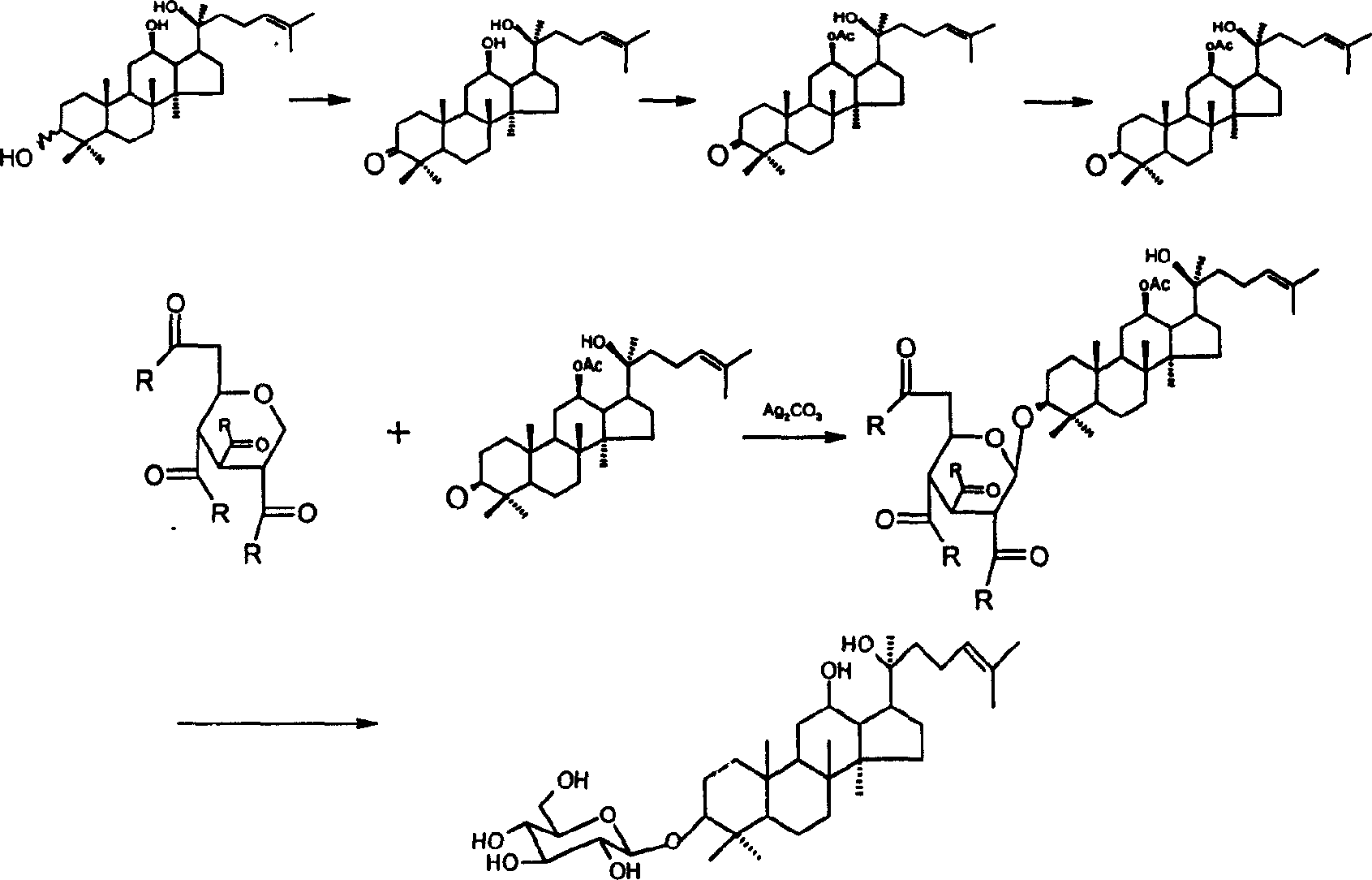
[0038] Example 1 Monosubstituted protopanaxadiol Synthesis of (A2)
[0039] (1) R' is benzoyl (Bz) (ie 12-benzoyl-protopanaxadiol)
[0040] Protopanaxadiol (A1) [prepared by the Chinese invention patent (patent application number: 200410018038.8)] 40g (0.087mol) was dissolved in pyridine (600ml), 44.51g (0.261mol) of benzoyl chloride was added at 0°C, 25 Stir at ℃ overnight, check by thin layer chromatography, the reaction is complete, add methanol to stop the reaction, dissolve in ethyl acetate after concentration, wash with saturated NaCl aqueous solution until neutral, and dry. After filtration, concentrated, purified by column chromatography [gradient elution: volume ratio of petroleum ether and ethyl acetate from 6:1 to 3:1] to obtain compound (A2-1) 41.07g, yield 84.3%, determined by HPLC The purity was 93.63%.
[0041] The physicochemical data of compound (A2-1) are as follows:
[0042] 1 H NMR (300MHz, CDCl 3 ): δ7.99-7.37(m, 5H), 5.2(m, 1H), 5.13(t, 1H), 3.9(dd...
Embodiment 2
[0059] Example 2 Fully protected D-glucose Synthesis of (B2)
[0060] (1) R is benzoyl (ie 1,2,3,4,6-penta-O-benzoyl-D-glucose)
[0061] D-glucose (150g, 0.833mol) was dissolved in 1650ml of anhydrous pyridine, 532.5ml (4.575mol) of benzoyl chloride was added at 0°C, stirred at room temperature overnight, detected by thin layer chromatography, after the reaction was complete, poured into a large amount of soaked in water until solidified, washed with water, and dried to obtain a white solid, that is, compound (B2-1) 552.3 g with a yield of 94.9% and a purity of 97.21% determined by HPLC. The physicochemical data are in agreement with literature values: Eagle, Andrew J.; et al, J. Chem. Res., 1993, 10, 2663-2679. (Refer to R.K.Ness, et al, J.Amer.Chem.Soc., 1951, 296-299 for the synthetic method).
[0062](2) R is an acetyl group (ie 1,2,3,4,6-penta-O-acetyl-D-glucose)
[0063] D-glucose (50g, 28mmol), anhydrous sodium acetate (25g) were added with acetic anhydride (350ml)...
Embodiment 3
[0073] Example 3 Glucose based donor Synthesis of (B3)
[0074] (1) R is benzoyl, X is OC(NH)CCl 3
[0075] a. Compound (B2-1) 120g (0.146mol) was dissolved in 600ml N,N-dimethylformamide, 20.6ml (0.36mol) of glacial acetic acid was added under stirring at room temperature, and 20.16ml of hydrazine hydrate was added dropwise at 0°C ( 0.36 mol), stirring at room temperature. TLC detection, the reaction was completed, purified by column chromatography [gradient elution: the volume ratio of petroleum ether and ethyl acetate from 5:1 to 3:1] to obtain the structural formula: The white solid was 66 g, the yield was 64.97%, and the purity determined by HPLC was 95.89%. Its physicochemical data agree with literature values: Mikamo, Masatomo; Carbohydr. Res., 1989, 191, 150-153.
[0076] b. 59 g (0.095 mol) of the compound obtained in the previous step was added with 150 ml of anhydrous dichloromethane and stirred to dissolve the solid. Under argon protection, 17.37 ml (0.171 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com