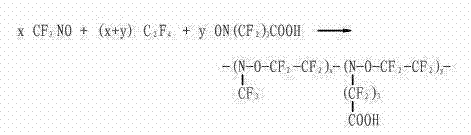Carboxyl nitroso-fluoro rubber (CNR) solution polymerization process
A technology of carboxynitroso fluorine and rubber solution, applied in the field of organic fluorine chemistry, can solve the problems of polymer separation difficulties, heat transfer, mass transfer deterioration, temperature out of control, etc., to avoid explosion hazards, easy separation, and improve mass transfer Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Replace the polymerization kettle with nitrogen and then evacuate it, pass through the jacket with ethanol at -65°C to circulate the cooling medium, cool the polymerization kettle to -30°C, and use the vacuum state of the polymerization kettle to add nitrosoperfluoro one by one by suction Butyric acid 100g, dichloromethane 4.6kg. Turn on the agitator of the polymerization kettle, and continue to feed -65°C ethanol into the jacket as a circulating cold medium. When the temperature of the polymerization kettle reaches -45°C, add 3.0 kg of nitrosotrifluoromethane. resulting in an increase in the temperature of the kettle. Due to the continuous refrigeration of the circulating cold medium, the temperature of the kettle dropped again. When the temperature in the reactor dropped to -55°C, 3.1kg of tetrafluoroethylene was added.
[0023] After the feeding was completed, the temperature of the polymerization kettle was allowed to rise naturally, and when the temperature inside...
Embodiment 2
[0026] The polymerization kettle was evacuated after being replaced with nitrogen, and -70°C ethanol was passed through the jacket to circulate the cold medium, and the polymerization kettle was cooled to -40°C. Utilizing the vacuum state of the polymerization kettle, 100 g of nitrosoperfluorobutyric acid and 5.0 kg of dichloromethane were sequentially added by suction. Turn on the agitator of the polymerization kettle, and continue to feed -70°C ethanol into the jacket to circulate the cold medium. resulting in an increase in the temperature of the kettle. Due to the continuous cooling of the circulating low-temperature cold medium, the temperature of the kettle dropped again. When the temperature in the reactor dropped to -60°C, 3.3 kg of tetrafluoroethylene was added.
[0027] After the feeding was completed, the temperature of the polymerization kettle was allowed to rise naturally, and when the temperature inside the kettle rose to -45°C, the terpolymerization reaction s...
Embodiment 3
[0030] The polymerization kettle was evacuated after being replaced with nitrogen, and a low-temperature cold medium of ethanol at -68°C was introduced into the jacket to cool the polymerization kettle to -35°C. Utilizing the vacuum state of the polymerization kettle, 110 g of γ-nitrosoperfluorobutyric acid and 5.5 kg of dichloromethane were sequentially added by suction. Turn on the agitator of the polymerization tank, and continue to feed -68°C ethanol as a low-temperature cold medium into the jacket. When the temperature of the polymerization tank reaches -45°C, add 3.1 kg of nitrosotrifluoromethane. resulting in an increase in the temperature of the kettle. Due to the continuous refrigeration of the circulating low-temperature cold medium, the temperature of the kettle dropped again. When the temperature in the reactor dropped to -65°C, 3.5 kg of tetrafluoroethylene was added.
[0031] After the feeding was completed, the temperature of the polymerization kettle was allow...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com