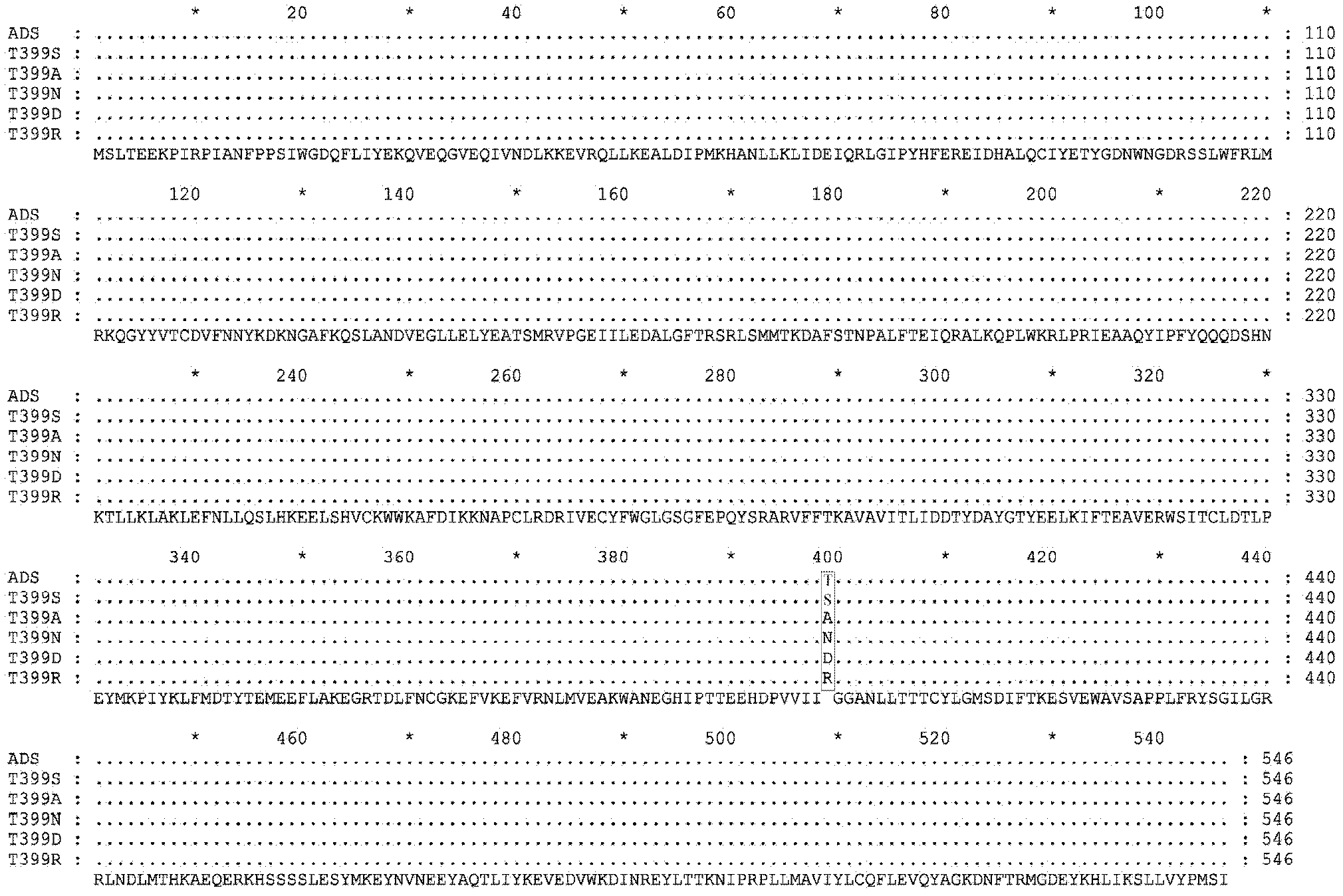Artemisia annua amorpha-4,11-diene synthase mutant with improved enzyme activity and application thereof
A kind of amorphadiene and mutant technology, which is applied in the direction of application, lyase, and introduction of foreign genetic material by using vectors, which can solve the problems of high cost and complicated artemisinin process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1, Extraction of Artemisia annua total RNA, PCR amplification of target gene ADS and site-directed mutagenesis
[0082] a. Extraction of Artemisia annua Total RNA
[0083] Artemisia annua material (about 100 mg) was thoroughly ground in liquid nitrogen. Transfer to a 1.5ml centrifuge tube, add 1mL Trizol (Invitrogen, Cat. 15596-018), mix well, and stand at room temperature for 5min. Centrifuge at 12,000rpm for 10min and discard the precipitate. Add 200 μL of chloroform to the supernatant, mix well, and centrifuge at 12,000 rpm for 10 min. Take the supernatant and add 500 μL of isopropanol to precipitate the RNA. Centrifuge at 12,000rpm for 10min, wash the pellet with 70% ethanol, dry in vacuum, dissolve in 20-50μL H 2 O (RNase free). Properly dilute the RNA with 10mM Tris-HCl (pH7.5), and measure the UV absorption value at a wavelength between 200-300nm. RNA concentration = 40 μg / mL × A 260 ×Dilution factor. A 260 / A 280 Should be between 1.9 and 2.1. ...
Embodiment 2
[0110] Embodiment 2, vector construction and Escherichia coli transformation
[0111] a. Vector construction
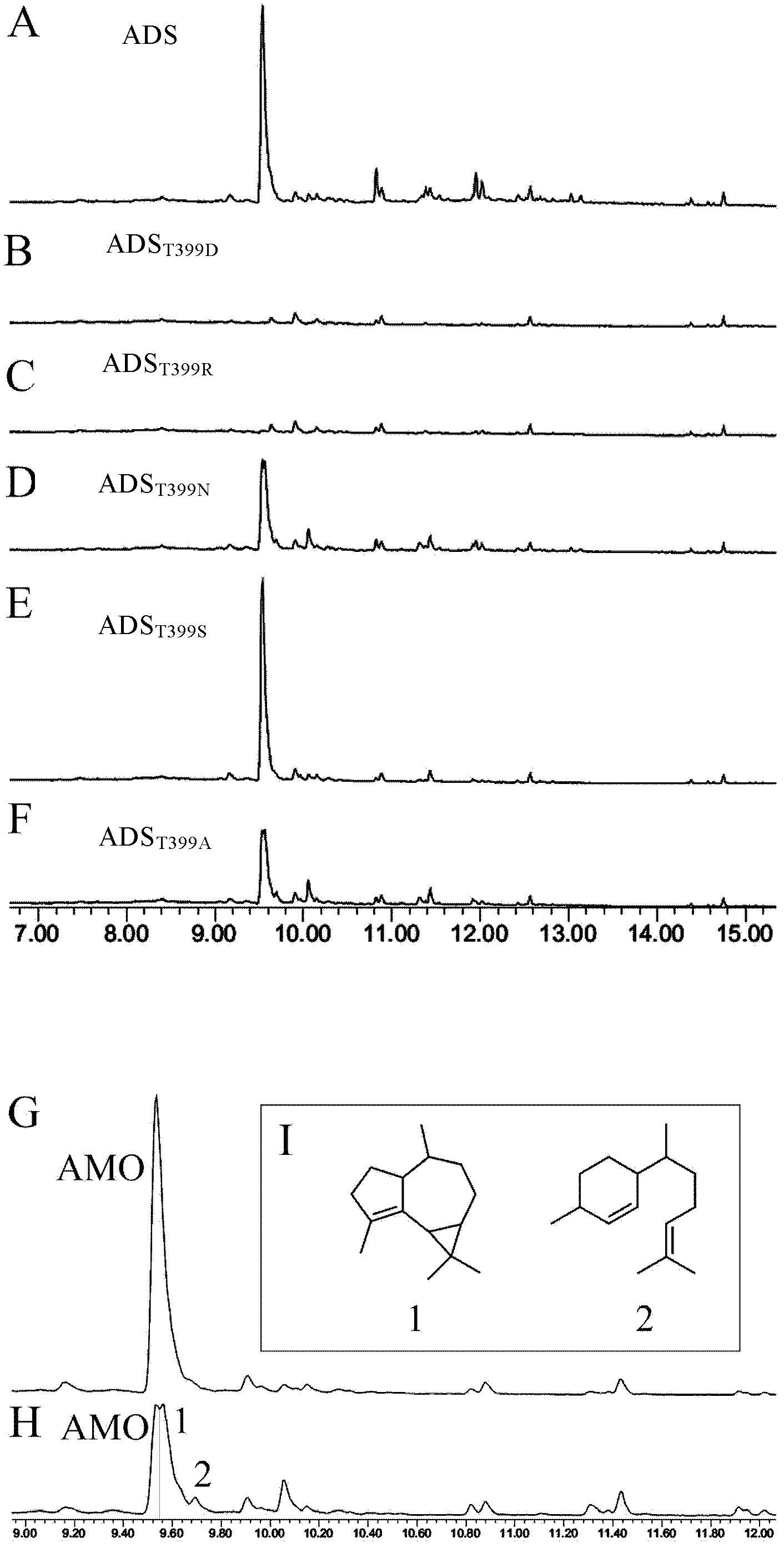
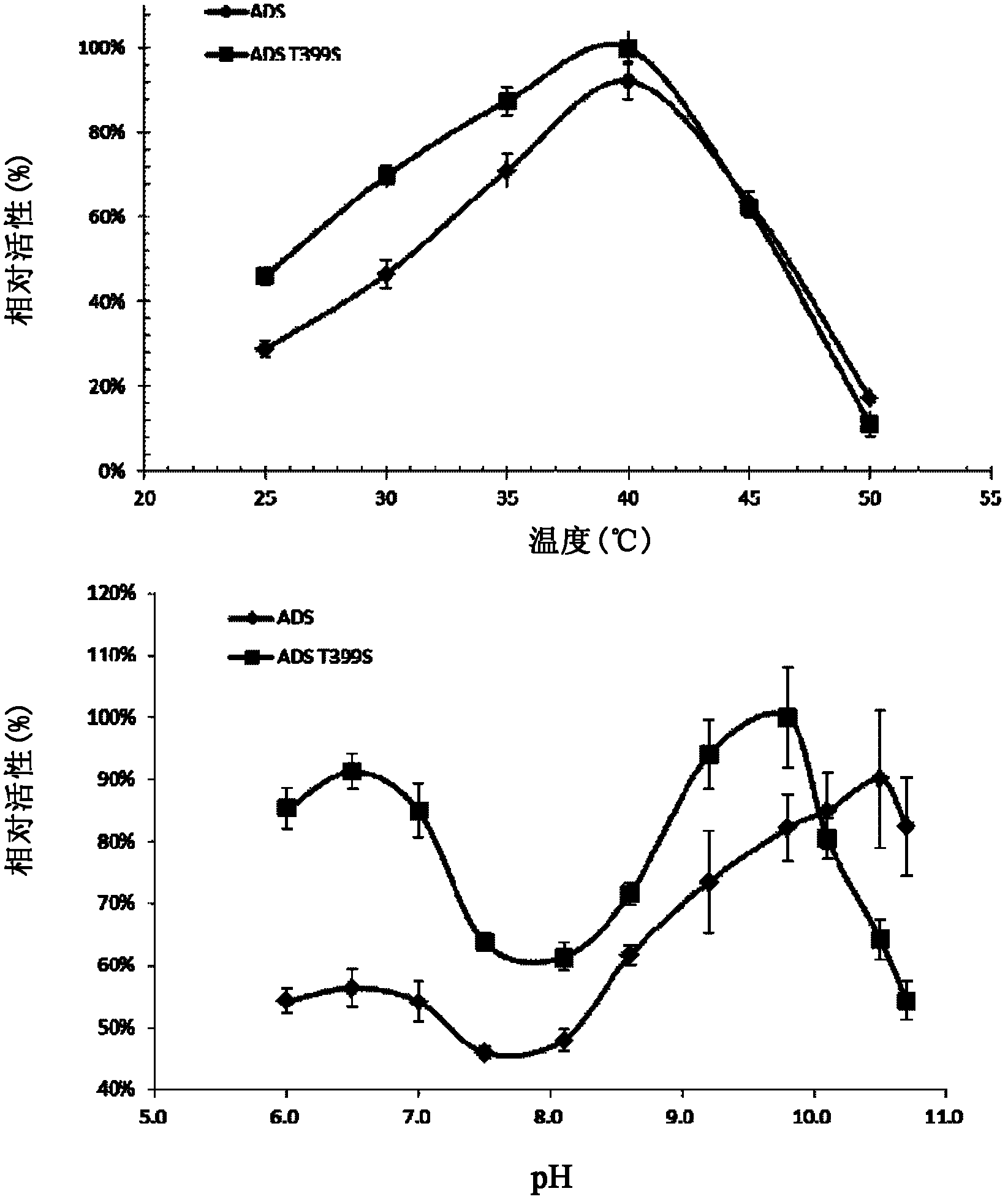
[0112] ADS coding region fragment (ADS) and mutant fragments (ADS-T399S, ADS-T399A, ADS-T399N, ADS-T399D, ADS-T399R) were amplified by KOD-plus DNA polymerase, and ligated into pET by NcoI / BamHI digestion -32a carrier (Amp r , Novagen, America), to obtain the recombinant vector.
[0113] b. Competent cell preparation
[0114] Escherichia coli DH5α or BL-21 stored at -70°C were streaked on a solid LB plate and cultured overnight at 37°C; a single colony was picked and cultured in 5 mL of liquid LB medium at 250 rpm overnight. The next day, scale up and inoculate into 500mL liquid LB medium at a ratio of 1 / 50, culture at 18-22°C until OD 600 ≈0.5 (about 5-6h), cooling on ice for 10min. Centrifuge at 2,500 g for 10 min at 4°C, resuspend the cells with 160 mL of transformation buffer, discard the supernatant, and finally resuspend the cells with 40 mL of transformati...
Embodiment 3
[0120] Embodiment 3, RT-PCR
[0121] 1 μg total RNA, Oligo(dt) 20 As primers, 10 μL reaction system was used for reverse transcription according to the requirements of the reverse transcription kit (TOYOBO, Osaka, Japan). React at 42°C for 30 minutes. After boiling water for 5 minutes, place on ice. The reverse transcription product (or diluted 10 times) can be directly used for PCR detection.
[0122] According to the known ADS sequence, PCR primers were synthesized. When analyzing the expression of ADS, Artemisia annua Actin1 (GenBank: EU531837) was used as an internal control to correct the amount of template in the RT-PCR reaction. The PCR conditions are as follows: the annealing temperature and extension time of the reaction are determined by the primers and the length of the amplified fragment. The general reaction conditions are: denaturation at 94°C for 5 min; denaturation at 94°C for 30 s, annealing at 55-60°C for 30 s, extension at 72°C for 30 s, amplification fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com