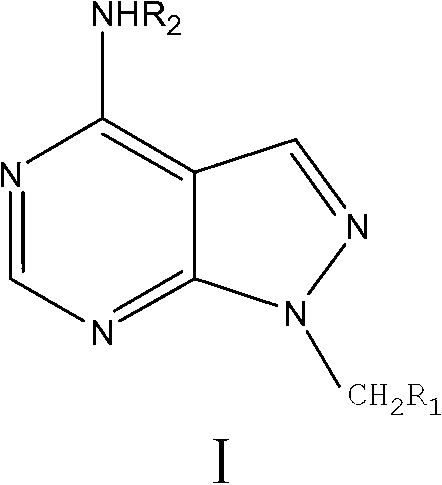
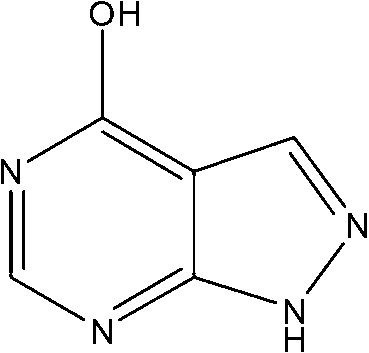
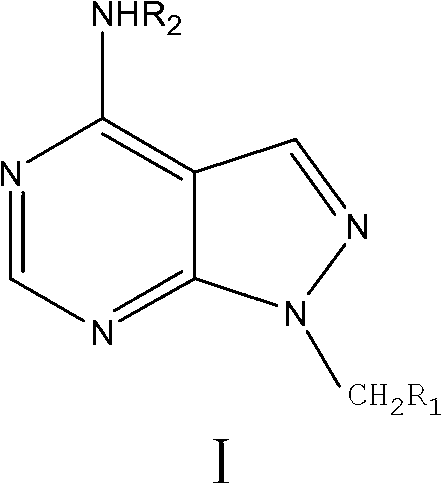
Allopurinol derivative and preparation method and application thereof
A technology for medicines and compounds, applied in the field of allopurinol derivatives and their preparation, achieves the effects of inhibiting activity, inhibiting tumor growth, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Synthesis of 1-N-ethyl-4-hydrazino-1H-pyrazol[3,4-d]pyrimidine (Compound 4 for short)
[0034]
[0035] (1) Synthesis of 4-chloro-1-ethyl-1H-pyrazol[3,4-d]pyrimidine (Compound 3 for short)
[0036] Dissolve 4-chloro-1H-pyrazolo[3,4-d]pyrimidine (0.1g, 0.65mmol) in 5mL of dry DMF, stir the reaction solution at room temperature for 10min, and slowly add a good amount with a dropping funnel triethylamine (0.20 g, 1.95 mmol), and the mixture was stirred at room temperature for 30 min. Bromoethane (0.084 g, 0.78 mmol) dissolved in 3 mL of dry DMF was slowly added dropwise to the mixture, stirring was continued for 1 h and then a catalytic amount of KI was added. The reaction solution was detected by TLC until complete reaction was detected, the mixture was poured into 15 mL of water, and the pH was adjusted to acidic with dilute hydrochloric acid, then extracted with ethyl acetate (4×20 mL), the organic layer was washed with saturated sodium chloride, anhydrous...
Embodiment 2
[0041] Example 2 (N-((4-chloroacetamido) phenyl)-4-amino-1H-pyrazol[3,4-d]pyrimidine-N-1-)-2-ethyl acetate (compound for short 8e) Preparation
[0042]
[0043] (1) Synthesis of ethyl (4-chloro-1H-pyrazol[3,4-d]pyrimidine-N-1-)-2-acetate (Compound 6 for short)
[0044] Slowly add TEA (1.78g, 17.62mmol) into 4-chloro-1H-pyrazolo[3,4-d]pyrimidine (0.81g, 5.26mmol) dissolved in 20mL dry DMF, stir at room temperature for 30min, Ethyl bromoacetate (1.06 g, 6.39 mmol) dissolved in 5 mL of dry DMF was slowly added dropwise to the mixture and stirring was continued for 2 h. The reaction solution was detected by TLC and poured into 30mL of water after the reaction was complete. After adjusting the pH value to acidic with dilute hydrochloric acid, it was extracted with ethyl acetate (3×25mL). The organic layer was washed with saturated sodium chloride and concentrated to obtain flocs . The crude product was subjected to silica gel column chromatography and eluted with petroleum et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com