B-cell epitope peptide of heart fatty acid binding protein (H-FABP), antibody and applications thereof
A fatty acid combination and B cell technology, applied in the field of medical testing, can solve problems such as no related reports, and achieve good antigenicity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
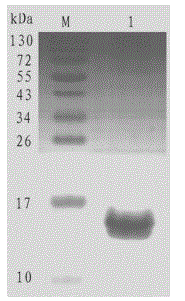
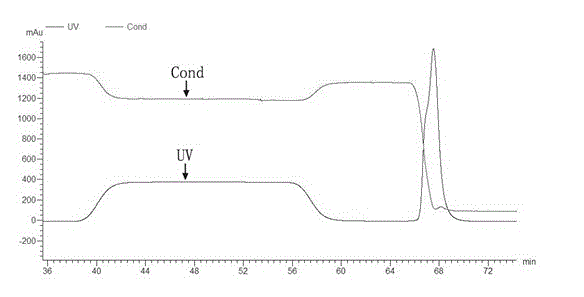
[0025] Embodiment 1, the preparation of H-FABP recombinant protein
[0026] Amplify the nucleotide sequence of the H-FABP gene coding region by reverse transcription PCR, clone it into the prokaryotic expression vector pET28a, induce protein expression after sequencing and identification, and purify the prepared H-FABP recombinant protein after characterization. The recombinant protein can be used It is used to prepare the immunogen and screening source of polyclonal antibodies, and can also be used as a calibrator in the subsequent establishment of H-FABP detection, specifically:
[0027] According to the reported gene sequence of heart type fatty acid-binding protein (heart type fatty acid-binding protein, H-FABP) (Genbank: NM_004102), the nucleotide sequence of its coding region is shown in SEQ ID NO.1. The corresponding polypeptide sequence after translation into amino acids is shown in SEQ ID NO.2, which encodes 133 amino acids, specifically:
[0028] MVDAFLGTWK LVDSKN...
Embodiment 2
[0049] Embodiment 2, the preparation of the B cell epitope peptide of H-FABP
[0050] According to the amino acid sequence of H-FABP, using the Protean software in the DNAStar Lasergene software package, the hydrophilicity, antigenic index and flexible region in the secondary structure of H-FABP were analyzed, and combined with the antigenic index prediction method to predict its B cell epitope. And use Chou & Fasman to predict β-turn angle, Emini method to predict antigen surface accessibility, Karplus & Schulz method to predict protein flexibility, Kolaskar & Tongaonkar protein antigenicity analysis, Parker method protein hydrophobicity analysis and Bepipred linear epitope prediction and other technical parameters, Screening the B cell epitope peptide of H-FABP, specifically amino acid residues at positions 32-47 in SEQ ID NO.2 (QVASMTKPTTIIEKNG, SEQ ID NO.5), in order to improve the interaction between the B cell epitope peptide and the carrier protein Linking ability, a...
Embodiment 3
[0053] Example 3, Preparation and Identification of Monoclonal Antibody
[0054] one, Immunization of Balb / c Mice with B Cell Epitope Peptide Derivatives
[0055] The H-FABP' stored in a -80°C refrigerator in Example 1 was taken out as an antigen, dissolved and filtered. Then select 6-week-old female Balb / c mice weighing about 20 g for immunization. For the first immunization, H-FABP' was emulsified and mixed with an equal volume of Freund's complete adjuvant, and the antigen was emulsified by double syringe push method to obtain the antigen mixture. Each mouse was injected with 100 μg of antigen mixture; the second and third immunizations were carried out on the 14th and 28th days after injection, respectively, the same antigen was used for immunization, the adjuvant was changed to incomplete Freund's adjuvant, and the amount of antigen, injection volume and route were different. Change. After the third immunization, the titer was measured by indirect ELISA method, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com