Asiatic acid salt tablet and preparation method thereof
A technology of oxalate tablet and oxalate tromethamine, which is applied in the directions of pill delivery, pharmaceutical formulations, and medical preparations containing active ingredients, can solve problems such as unsatisfactory drug efficacy and low bioavailability, and achieve Good bioavailability, easy operation, good particle fluidity and compressibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
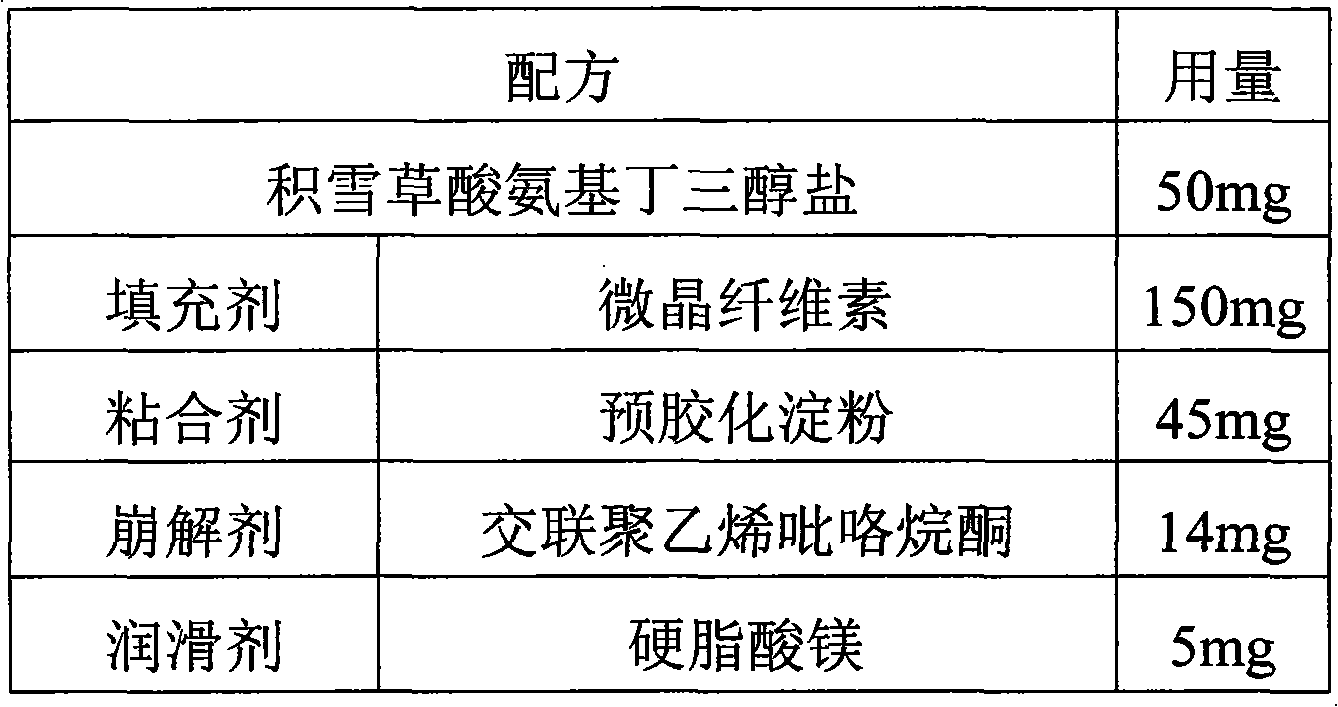
[0026]
[0027] Preparation method: according to the above formula, uniformly mix crushed and sieved asiatic acid tromethamine salt, microcrystalline cellulose, pregelatinized starch and cross-linked polyvinylpyrrolidone, then mix with 5% ethanol solution, and granulate , dried, and then mixed with a lubricant and compressed into tablets.
[0028] Wherein, said asiatic acid tromethamine salt is crushed and sieved to pass through a 60-mesh sieve; said microcrystalline cellulose, pregelatinized starch and cross-linked polyvinylpyrrolidone are crushed and sieved to pass through a 80-mesh sieve; The particle size of the granulated particles is 20 mesh; the drying temperature is preferably 90°C to control the water mass percentage within 3%.
[0029] After testing (rat tail vein injection method, see "Journal of Southern Medical University" 2009 06), the bioavailability of asiatic acid tromethamine salt in the obtained tablet is 17.9%.
Embodiment 2
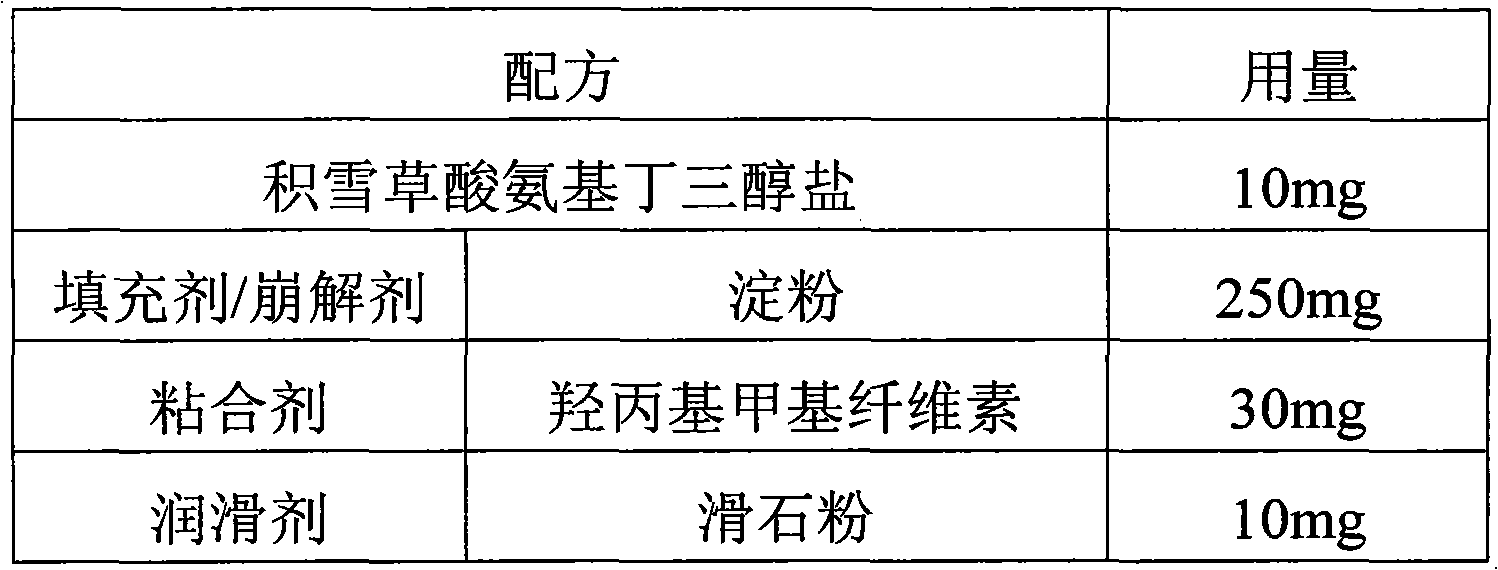
[0031]
[0032] Preparation method: according to the above formula, uniformly mix crushed and sieved asiatic acid tromethamine salt, four-fifths of starch, and hydroxypropyl methylcellulose, then mix with 20% ethanol solution, granulate, Dry it, then mix it with the remaining starch and lubricant, and press it into tablets.
[0033]Wherein, said asiatic acid tromethamine salt is crushed and sieved to pass through a 100 mesh sieve; said starch and hydroxypropyl methylcellulose are crushed and sieved to pass through a 100 mesh sieve; said granulated granules The particle size is 50 mesh; the drying temperature is preferably 50°C to control the water mass percentage within 3%.
Embodiment 3
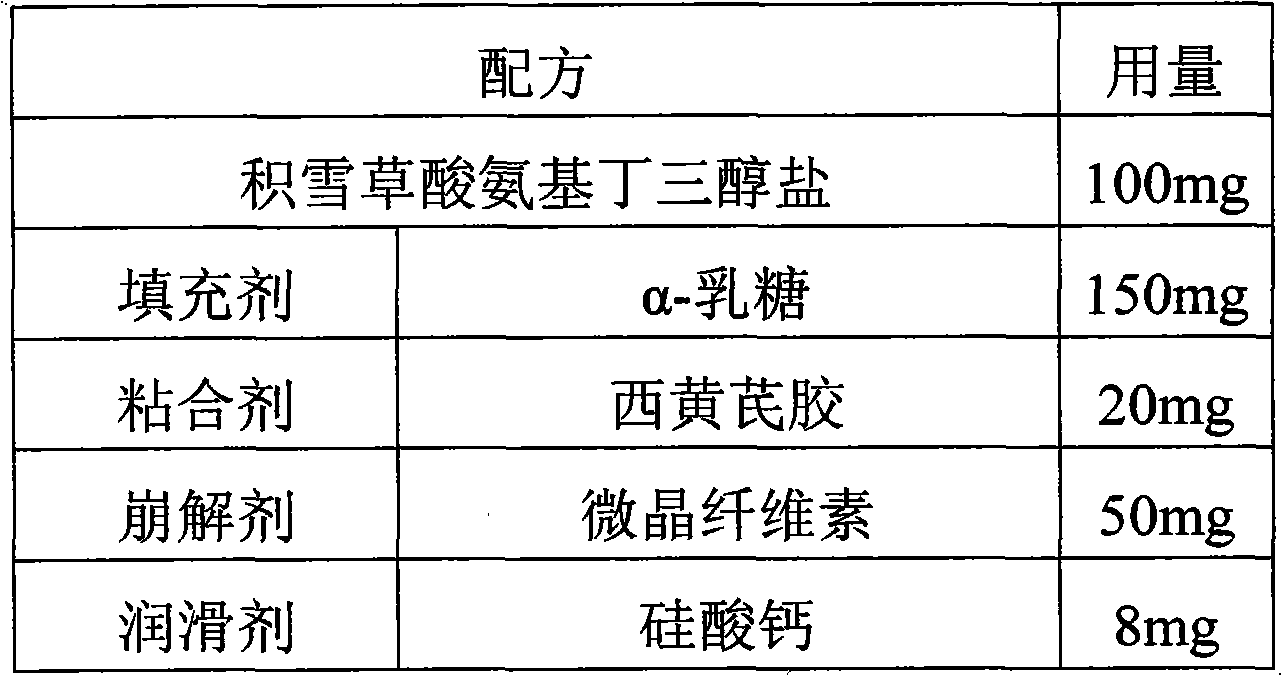
[0035]
[0036] Preparation method: according to the above formula, uniformly mix crushed and sieved asiatic acid tromethamine salt, microcrystalline cellulose, α-lactose and tragacanth gum, then mix with 20% ethanol solution, granulate, and dry. Afterwards, it is mixed with a lubricant and compressed into tablets.
[0037] Wherein, said asiatic acid tromethamine salt is crushed and sieved to pass through a 60-mesh sieve; said microcrystalline cellulose, α-lactose and tragacanth gum are crushed and sieved to pass through a 80-mesh sieve; The particle size of the granules is 20 mesh; the drying temperature is preferably 50°C to control the water mass percentage within 3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com