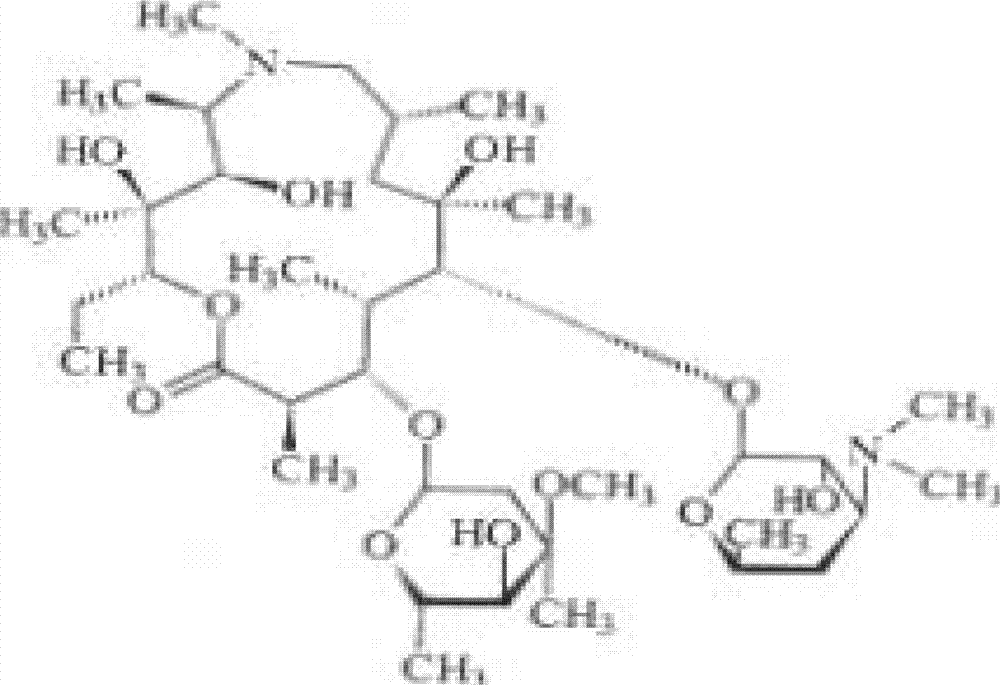
Lyophilized preparation of citric acid and azithromycin, and preparation method thereof
A technology of azithromycin citrate and freeze-dried preparations, applied in the field of medicine, can solve the problems of harsh process conditions, high cost, easy to be decomposed and damaged, etc., and achieves the effects of good stability, shortening freeze-drying time, and reducing energy consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
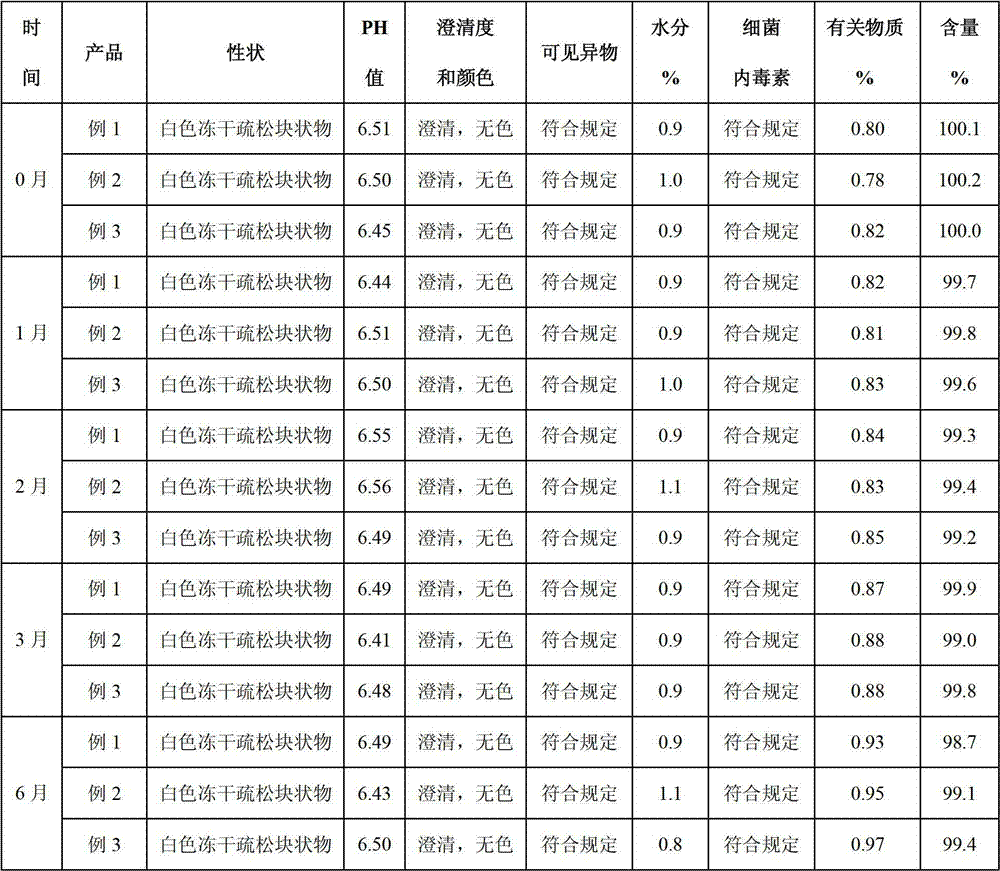
Examples
Embodiment 1
[0020] 1) Prepare citric acid solution: dissolve 200g citric acid in 500ml water for injection (cool to below 45°C), the concentration is 2.0mol / L; 2) Prepare sodium hydroxide solution: inject 56g sodium hydroxide in 500ml Dissolve in water (cooled to below 45°C), the concentration is 3mol / L; 3) Prepare azithromycin suspension: put 250g of azithromycin in a sterile batching tank, add 500ml of water for injection (cooled to below 45°C), and prepare Azithromycin suspension; 4) Slowly add the citric acid solution prepared in step 1) into the azithromycin suspension, stir to make it fully react; use the sodium hydroxide solution prepared in step 2) to adjust the pH to 6.50, and use the remaining prescription amount Dilute the mixed solution to the mark with water for injection (cooled to below 45°C); 5) Add medicinal activated carbon with a mass volume ratio of 0.04% to the solution in step 4), stir for 15 minutes, decarbonize and filter with a 0.45um filter membrane , and then us...
Embodiment 2
[0024] 1) Preparation of citric acid solution: Dissolve 250g of citric acid in 500ml water for injection (cooled to below 45°C), the concentration is 2.6mol / L; 2) Preparation of sodium hydroxide solution: Inject 75g of sodium hydroxide in 500ml Dissolve in water (cooled to below 45°C), the concentration is 3.7mol / L; 3) Prepare azithromycin suspension: put 250g of azithromycin in a sterile batching tank, add 500ml of water for injection (cooled to below 45°C), and prepare 4) Slowly add the citric acid solution prepared in step 1) into the azithromycin suspension, stir to make it fully react; use the sodium hydroxide solution prepared in step 2) to adjust the pH to 7.00, and use the remaining prescription Dilute the mixed solution to the mark with a certain amount of water for injection (cooled to below 45°C); 5) Add 0.04% mass volume ratio of medicinal activated carbon to the solution in step 4), stir for 20 minutes, and decarbonize and filter with a 0.45um filter membrane Fina...
Embodiment 3
[0028] 1) Prepare citric acid solution: dissolve 375g citric acid in 500ml water for injection (cool to below 45°C), the concentration is 3.9mol / L; 2) Prepare sodium hydroxide solution: inject 90g sodium hydroxide with 500ml Dissolve in water (cooled to below 45°C), the concentration is 4.5mol / L; 3) Prepare azithromycin suspension: put 250g of azithromycin in a sterile batching tank, add 500ml of water for injection (cooled to below 45°C), and prepare into azithromycin suspension; 4) Slowly add the citric acid solution prepared in step 1) into the azithromycin suspension, stir to make it fully react; use the sodium hydroxide solution prepared in step 2) to adjust the pH to 7.50, and use the remaining prescription Dilute the mixed solution to the mark with a certain amount of water for injection (cooled to below 45°C); 5) Add 0.04% mass volume ratio of medicinal activated carbon to the solution in step 4), stir for 25 minutes, and decarbonize and filter with a 0.45um filter memb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com