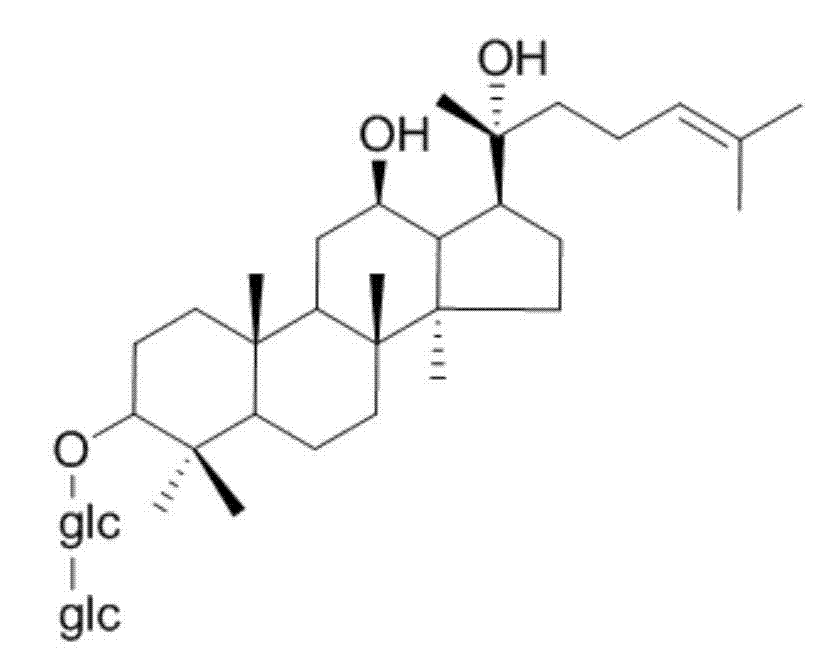
Method for preparing 20 (R)-ginseniside Rg3
A technology of ginsenosides and Panax notoginseng saponins, which is applied in the field of preparation of ginsenoside Rg3, can solve the problems of difficult industrial production, complex and expensive extraction and preparation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Preparation of the present invention 20(R)-ginsenoside Rg 3 The specific steps of the method are as follows:
[0015] (1) Acidification
[0016] Dissolve Panax notoginseng saponins in water, add acid (hydrochloric acid, phosphoric acid, sulfuric acid, acetic acid, glacial acetic acid, formic acid and other acids) to adjust the pH to 1~3, heat and reflux for 30~60 min, recover the acid solution and concentrate to a paste, dry A crude conversion product was obtained.
[0017] (2) Separation and purification by column chromatography
[0018] The crude conversion product obtained in step (1) was dissolved and adsorbed in methanol to 1.5 times the amount of silica gel to mix the sample, evaporated to dryness at room temperature, and subjected to silica gel column chromatography, eluted with a mixed solvent of chloroform:methanol:water gradient, and collected the eluate , with 20(R)-ginsenoside Rg 3 As a control, follow-up detection with thin-layer chromatography (TLC),...
Embodiment 2
[0026] First obtain the total saponins of notoginseng according to the method of Example 1, then dissolve 100 g of total saponins of notoginseng in 1000 ml of water, adjust the pH to 3 with hydrochloric acid, heat and reflux for 60 min, recover the acid solution and concentrate it to a paste, and dry to obtain crude Conversion product 101.2 g. The crude conversion product was dissolved in an appropriate amount of methanol, adsorbed on 150 g of 200-300 mesh silica gel, mixed with the sample, evaporated to dryness at room temperature, and subjected to silica gel column chromatography. Carry out gradient elution, collect eluate, with 20 (R)-ginsenoside Rg 3 As a control, follow-up detection with thin-layer chromatography (TLC), combined with 20(R)-ginsenoside Rg 3 Fractions, reclaim the solvent to 200 ml, let stand, filter, and dry the filter cake to obtain 20(R)-ginsenoside Rg 3 16.8 g. Detected by high performance liquid chromatography (HPLC), the resulting product 20(R)...
Embodiment 3
[0028] First obtain the total saponins of notoginseng according to the method of Example 1, then dissolve 100 g of total saponins of notoginseng in 1000 ml of water, adjust the pH to 1 with hydrochloric acid, heat and reflux for 50 min, recover the acid solution and concentrate it to a paste, and dry to obtain crude Conversion product 102.5 g. The crude conversion product was dissolved in an appropriate amount of methanol, adsorbed on 150 g of 200-300 mesh silica gel, mixed with the sample, evaporated to dryness at room temperature, and subjected to silica gel column chromatography. Carry out gradient elution, collect eluate, with 20 (R)-ginsenoside Rg 3 As a control, follow-up detection with thin-layer chromatography (TLC), combined with 20(R)-ginsenoside Rg 3 Fractions, reclaim the solvent to 200 ml, let stand, filter, and dry the filter cake to obtain 20(R)-ginsenoside Rg 3 15.9 g. Detected by high performance liquid chromatography (HPLC), the resulting product 20(R)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com