Method for expressing recombinant human bone morphogenetic protein in insect cell
A morphogenetic protein, insect cell technology, applied in animal/human proteins, biochemical equipment and methods, botanical equipment and methods, etc., can solve the problems of low protein expression, high treatment cost, and high preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016] Below in conjunction with embodiment, the present invention is further described. The following examples are illustrative rather than limiting, and the protection scope of the present invention cannot be limited by the following examples.
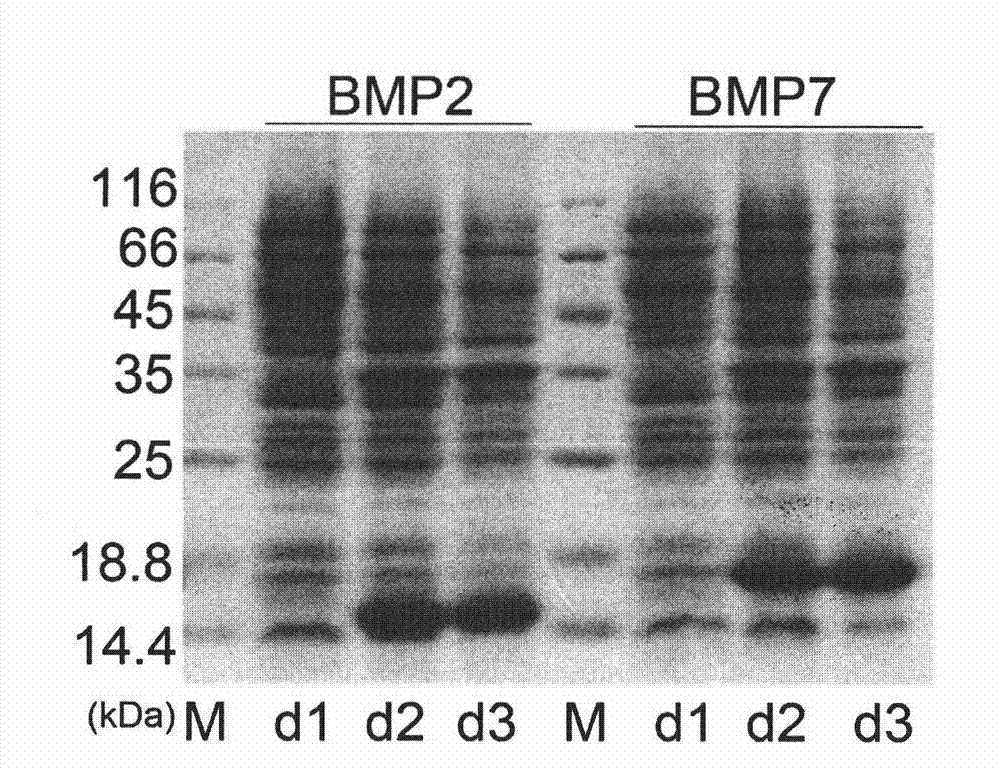
[0017] The steps of the method for highly expressing mature peptides of recombinant human bone morphogenetic proteins BMP2 and BMP7 in insect cells are as follows:
[0018] (1) Preparation and identification of recombinant baculovirus
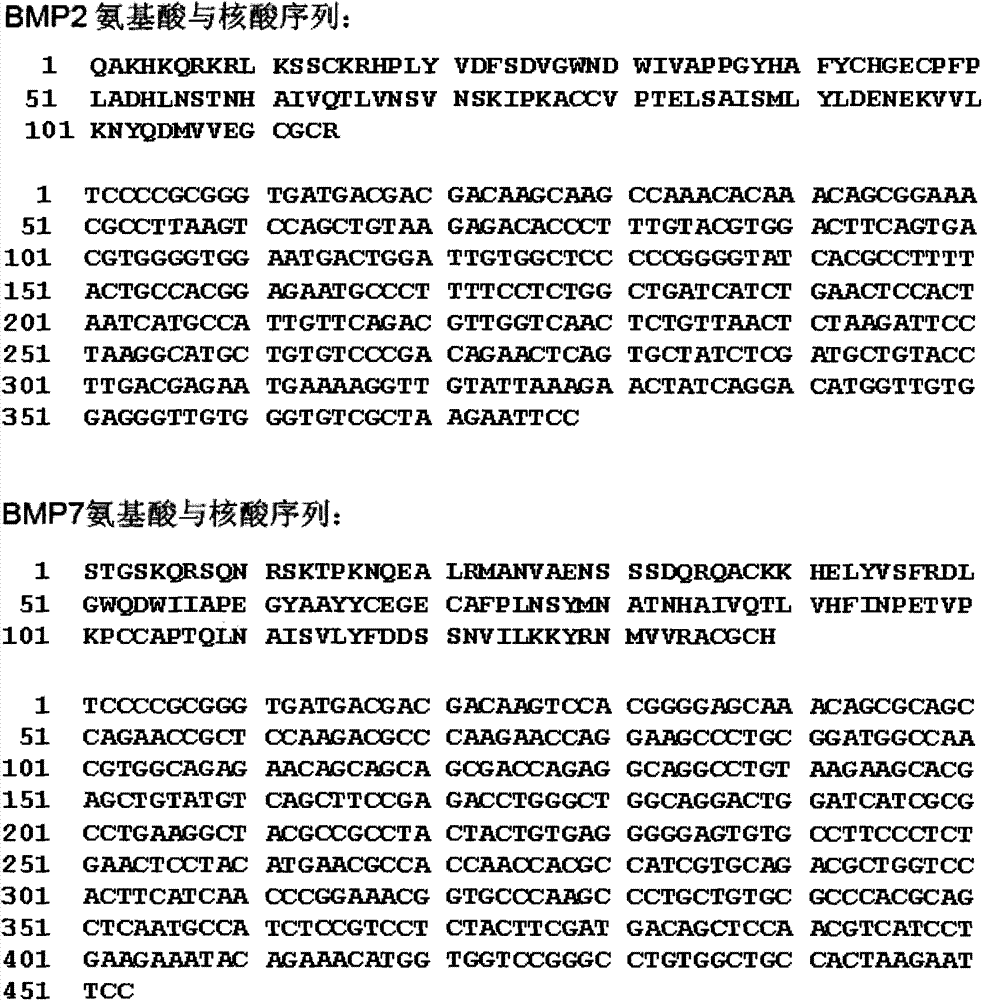
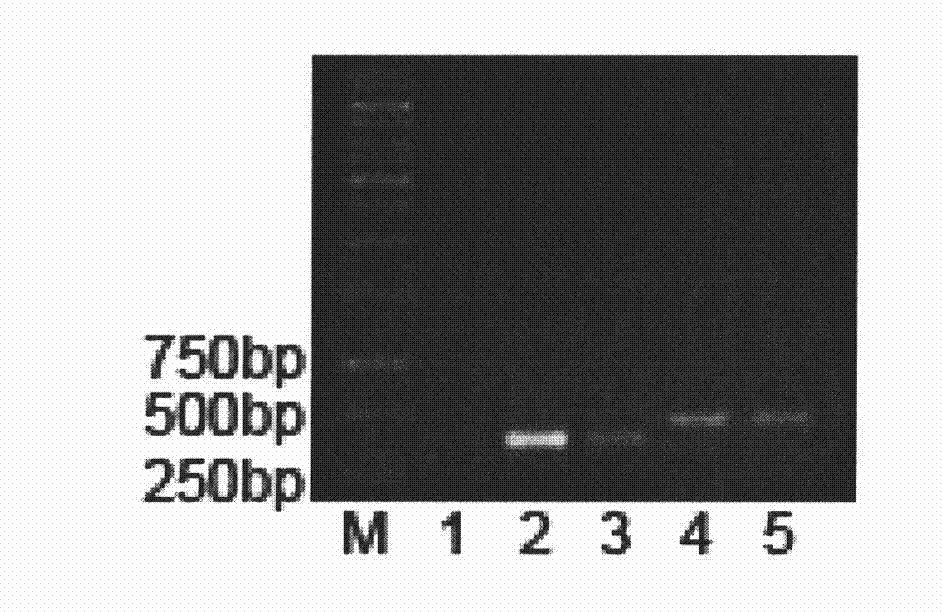
[0019] Respectively in human BMP2, BMP7 mature peptide ( figure 1 ) with a 6xHis tag added to the N-terminus, and its gene fragments were cloned into an improved insect-baculovirus expression vector with BamHI and EcoRI sites, and then co-transfected into sf9 insect cells. Clear culture fluid, lyse baculovirus with proteinase K, carry out PCR with viral genome DNA as a template, and amplify the DNA fragments encoding BMP2 and BMP7 mature peptide respectively ( figure 2 ), indicating that a recombi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com