Biosynthesis method for increasing accumulation of L-5-methyltetrahydrofolate
A technology of methyltetrahydrofolate and synthetase, which is applied in the field of metabolic engineering, can solve the problems of less research on the biosynthesis of L-5-methyltetrahydrofolate, improve the utilization rate of raw materials, reduce production costs and energy consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
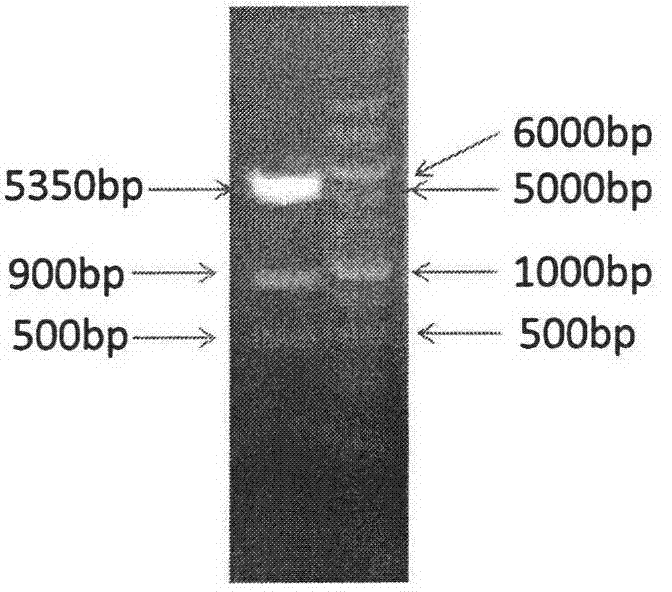
[0040] Example 1 Construction of co-expression plasmids
[0041] 1.1 Primer design
[0042] According to the folA and metF sequences reported by NCBI, the following 4 primers were designed:
[0043] folAUP: GAGCTC GGGATAATGATCAGTCTGATTGCGGC
[0044] fol ADOWN: AAGCTT TTACCGCCGCTCCAGAATCTCAA
[0045] metFUP: GGATCC ATGAGCTTTTTTCACGCCAGCCAGCG
[0046] metFDOWN: GAGCTC TTATAAACCAGGTCGAACCCCCA
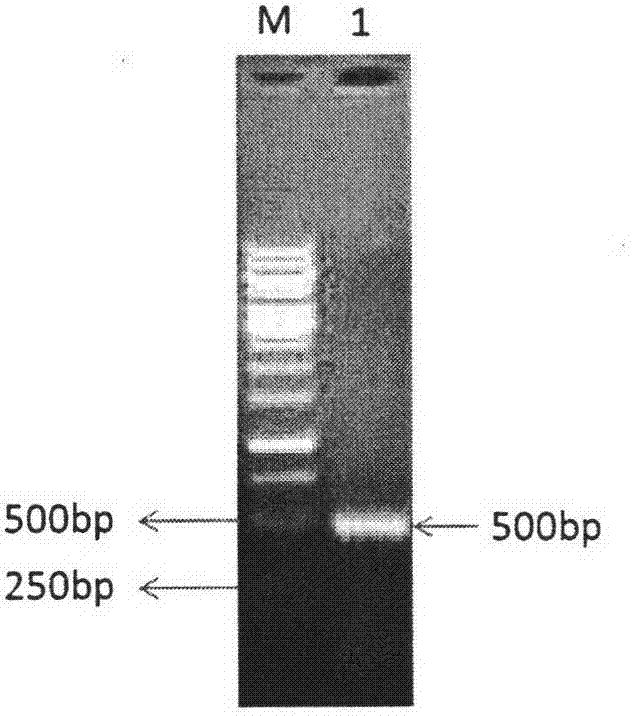
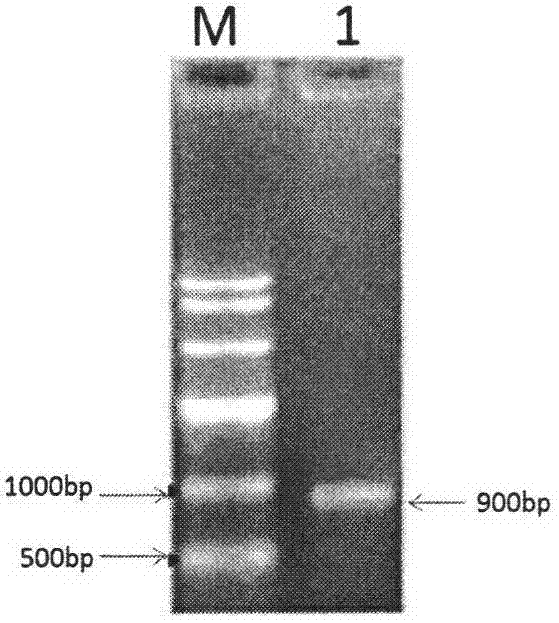
[0047] Among them, folAUP and folADOWN are used to amplify the folA coding region; metFUP and metFDOWN are used to amplify the metF coding region; the underlines on folAUP, folADOWN, metFUP, and metFDOWN represent the Sac I restriction enzymes introduced on folAUP, folADOWN, metFUP, and metFDOWN respectively site, Hind III restriction site, BamH I restriction site and Sac I restriction site.
[0048] 1.2 PCR amplification of folA and metF sequences
[0049] The genome of Escherichia coli E.coli BL21(DE3) was extracted.
[0050] Using the Escherichia coli E.coli BL21 (DE3) ...
Embodiment 2
[0069] Example 2 Transformation of Bacterial Strain Accumulating L-5-Methyltetrahydrofolate with Expression Plasmid
[0070] Plasmids pETfolAmetF, pETfolA, pETmetF and pET-28a(+) were respectively transformed into host bacteria E.coli BL21(DE3), respectively named PAF01, PA02, PF03 and P04, and coated with 20 μg / mL kanamycin Solid LB plates, cultivated at 37°C until the transformants grow out. Among them, E.coli BL21 (DE3) is a non-kanamycin-resistant strain and cannot grow on a solid LB plate containing kanamycin. Therefore, the transformants grown on the plate are transformed with pETfolAmetF, pETfolA, pETmetF and E. coli with pET-28a(+) plasmid.
[0071] Randomly pick the transformants of PAF01, PA02, PF03 and P04, shake the flask for fermentation culture, add inducer at an appropriate time, continue to culture for 3-8 hours, ultrasonically disrupt the cells, and perform SDS-PAGE on the soluble protein, the results are as follows: Figure 5 . according to Figure 5 Th...
Embodiment 3
[0074] Example 3 Fermentation of L-5-Methyltetrahydrofolate in PAF01, PA02, PF03 and E.coli RL21(DE3) Shake Flasks
[0075] Single colonies of PAF01, PA02, PF03 and E.coli BL21(DE3) (original bacteria, named BL21) grown overnight on solid LB plates at 37°C were respectively inserted into liquid LB medium and cultured in shake flasks overnight.
[0076] Take an appropriate amount of bacterial liquid and transfer it into the shake flask fermentation medium, and cultivate for 10 h. Among them, add the inducer at an appropriate time; add 0.05 g of precursor folic acid and 8% glycerol at an appropriate time, and take samples at 4h, 6h, 8h, and 10h of fermentation to detect the accumulation of L-5-methyltetrahydrofolate in the fermentation product, The test results are shown in Table 1.
[0077] According to the results in Table 1, the accumulation of L-5-methyltetrahydrofolate in PAF01, PA02, PF03, and BL21 reached the highest level at 10 h of fermentation, reaching about 11 ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com