A kind of multivesicular liposome containing exenatide and its preparation method and application
A multivesicular liposome and exenatide technology, which is applied to medical preparations containing active ingredients, liposome delivery, and medical preparations without active ingredients, etc., can solve the problems of frequent administration, poor patient compliance, Fast release speed and other problems, to achieve the effect of reducing adverse reactions, high drug loading concentration, and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
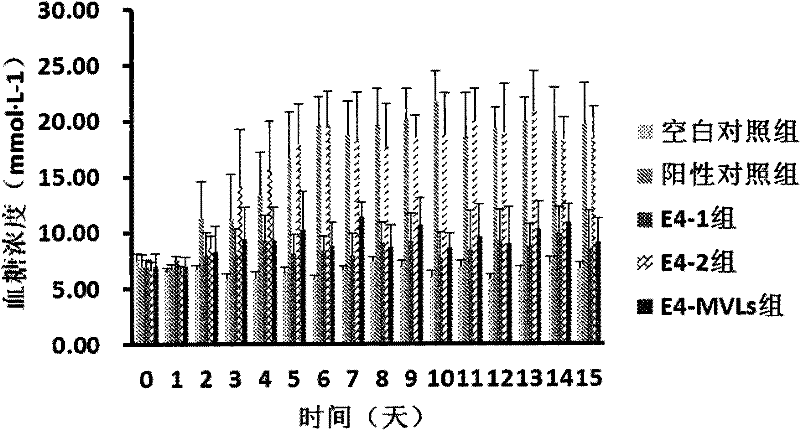
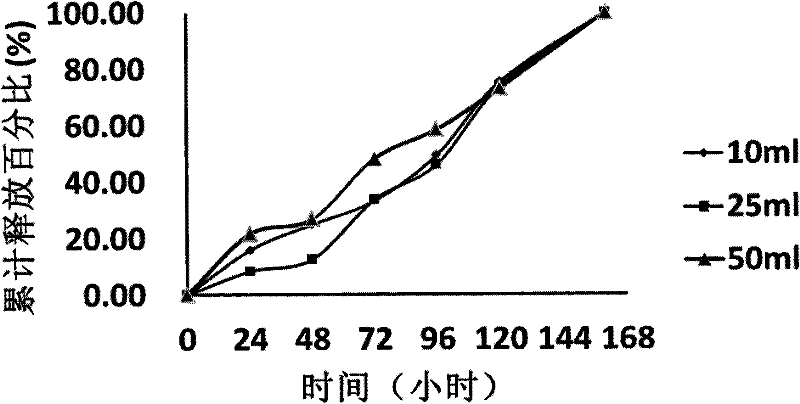
Image
Examples
Embodiment 1
[0055] Step 1: In a clean glass vessel add 1 mL containing 19.8mM (14.85mg) lecithin, 4.2mM (3.13mg) dipalmitoylphosphatidylglycerol, 30mM (11.61mg) cholesterol and 3.75mM (3.32mg) trioleic acid Chloroform solution of glycerides. This solution is called the lipid phase.
[0056] Step 2: Add 1 mL of the internal water phase of an aqueous solution of exenatide 1 mg, 20 mM (4.20 mg) citric acid and 2.5% (25 mg) sucrose into the above-mentioned glass container containing the lipid phase, and use a high-speed shear Stir at a speed of 10,000 rpm for 10 minutes to obtain W / O colostrum.
[0057] Step 3: Place 5 mL of an external aqueous phase solution containing 5.4% (w / v) (270 mg) glucose and 1% (50 mg) PVA on the colostrum layer, then mix at a speed of 10,000 rpm for 10 seconds to obtain W / O / W type double emulsion.
[0058] Step 4: Transfer the double emulsion to a 250mL Erlenmeyer flask containing 5mL containing 5.4% (w / v) (270mg) glucose and 1% (50mg) PVA, in a constant temper...
Embodiment 2
[0062] Step 1: Add 1 mL of 5 mM (3.96 mg) Hydrogenated Soy Lecithin, 0.5 mM (0.38 mg) Dipalmitate Phosphatidylserine, 10 mM (3.87 mg) Cholesterol and 0.155 mM (0.073 mg) Tricaprylic Glycerin to a clean glass container Esters in chloroform-ether (volume ratio 1:1) solution. This solution is called the lipid phase.
[0063] Step 2: Add 0.5 mL of the inner aqueous phase of 5 mg of exenatide, 20 mM (2.10 mg) of citric acid and 6% (30 mg) of sucrose into the above-mentioned glass container containing the lipid phase, and use high-speed shear The machine stirred at 10,000rpm for 10 minutes to obtain W / O type colostrum.
[0064] Step 3: 7.5 mL containing 5.4% (w / v) (405 mg) glucose and 1% (75 mg) gelatin in the outer aqueous phase solution is placed on the colostrum layer, then mixed for 40 seconds at a speed of 4500 rpm to obtain W / O / W type double emulsion.
[0065] Step 4: Transfer the double emulsion to a 250mL Erlenmeyer flask containing 9mL containing 5.4% (w / v) (486mg) gluc...
Embodiment 3
[0069] Step 1: Add 1 mL of a dichloromethane solution containing 100 mM (75 mg) lecithin, 20 mM (8.49 mg) phosphatidic acid, 10 mM (3.87 mg) cholesterol and 13 mM (11.51 mg) triolein into a clean glass container. This solution is called the lipid phase.
[0070] Step 2: Add 1 mL of the inner aqueous phase of the aqueous solution of exenatide 2.5 mg and 100 mM (21.01 mg) citric acid into the above-mentioned glass container containing the lipid phase, and stir at a speed of 10,000 rpm with a high-speed shear 10 minutes to get W / O type colostrum.
[0071] Step 3: 20 mL of an external aqueous phase solution containing 6% (w / v) (1200 mg) glucose and 1% (200 mg) hydroxymethyl starch was placed on the colostrum layer, then mixed at a speed of 10,000 rpm for 10 seconds, Get W / O / W type double milk.
[0072] Step 4: Transfer the double emulsion to a 250mL Erlenmeyer flask containing 5mL containing 6% (w / v) (300mg) glucose and 1% (50mg) hydroxymethyl starch, in a constant temperature w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com