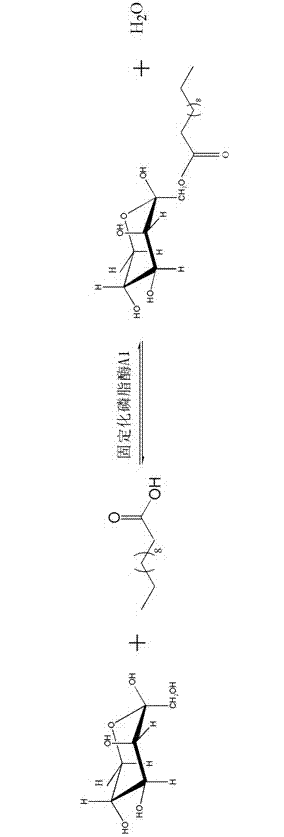
Method for catalytic synthesis of fructose lauric acid monoester by using immobilized phospholipase A1
A technology for immobilizing phospholipase and lauric acid monoester, applied in the field of fructose lauric acid monoester, can solve problems such as unseen, and achieve the effects of low toxicity, good industrial application prospect and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
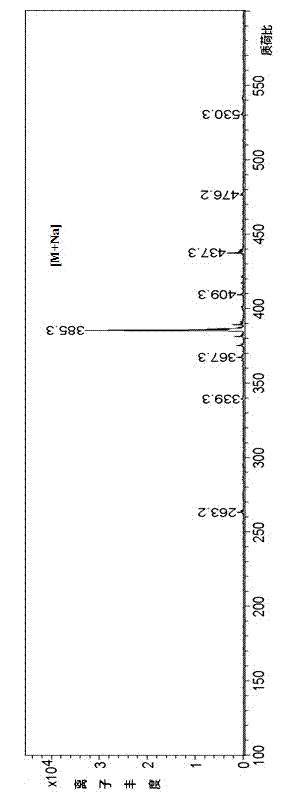
Image
Examples
Embodiment 1
[0025] a. Weigh 5 g of activated DA-201 macroporous adsorption resin, mix 10 g of phosphate buffer solution, and 1 g of free phospholipase A1, stir and adsorb at 25°C for 2 hours, and then filter to obtain macroporous adsorption resin immobilized phospholipase , its enzyme activity is 1850 U / g. Vacuum dried, ready for use;
[0026] b. Place the 4? molecular sieve in an oven at 105°C for 10 hours, then place it in a desiccator and cool it to room temperature for later use; after that, add the activated 4? in tert-butanol, sealed and placed for 72h, and filtered to remove molecular sieves to obtain a dehydrated organic solvent;
[0027] c. Enzymatic esterification: Mix 10g of lauric acid and 1.8g of fructose in the above-mentioned dehydrated organic solvent with a volume of 42ml, then add 2g of immobilized phospholipase A1 to start the reaction; after 2 hours of reaction, add 10g of activated 4? Generated water; shake or stir the reaction at 45°C for 48 hours, and the shakin...
Embodiment 2
[0029] a. Weigh 5 g of activated DA-201 macroporous adsorption resin, mix with 20 g of phosphate buffer, and 1 g of free phospholipase A1, stir and adsorb at 30°C for 4 hours, and then filter to obtain macroporous adsorption resin immobilized phospholipase. Its enzyme activity is 1462 U / g. Vacuum dried, ready for use;
[0030] b. Place the 4? molecular sieve in an oven at 100°C to activate for 8 hours, and then place it in a desiccator to cool to room temperature for later use; after that, add the activated 4? molecular sieve to the reaction medium in an amount of 60g per liter of solvent in tert-amyl alcohol, sealed for 24 hours, filtered to remove molecular sieves, to obtain a dehydrated organic solvent;
[0031] c. Enzymatic esterification: Mix 10g of lauric acid and 2.3g of fructose in the above-mentioned dehydrated organic solvent with a volume of 100ml, then add 2g of immobilized phospholipase A1 to start the reaction; add 10g of activated 4? Generated water; shake o...
Embodiment 3
[0033] a. Weigh 5 g of activated DA-201 macroporous adsorption resin, mix with 20 g of phosphate buffer, and 1 g of free phospholipase A1, stir and adsorb at 35°C for 3 hours, then filter to obtain macroporous adsorption resin immobilized phospholipase, Its enzyme activity is 1344 U / g. Vacuum dried, ready for use;
[0034] b. Place the 3? molecular sieve in an oven at 95°C to activate for 5 hours, and then place it in a desiccator to cool to room temperature for later use; after that, add the activated 3? molecular sieve to the reaction medium in an amount of 50 g per liter of solvent in acetone, sealed for 72 hours, and filtered to remove molecular sieves to obtain a dehydrated organic solvent;
[0035] c. Enzymatic esterification: Mix 40.6g of lauric acid and 36.5g of fructose in the above-mentioned dehydrated organic solvent with a volume of 812ml, then add 8.12g of immobilized phospholipase A1 to start the reaction; add 40.6g of activated 3? The water generated during ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com