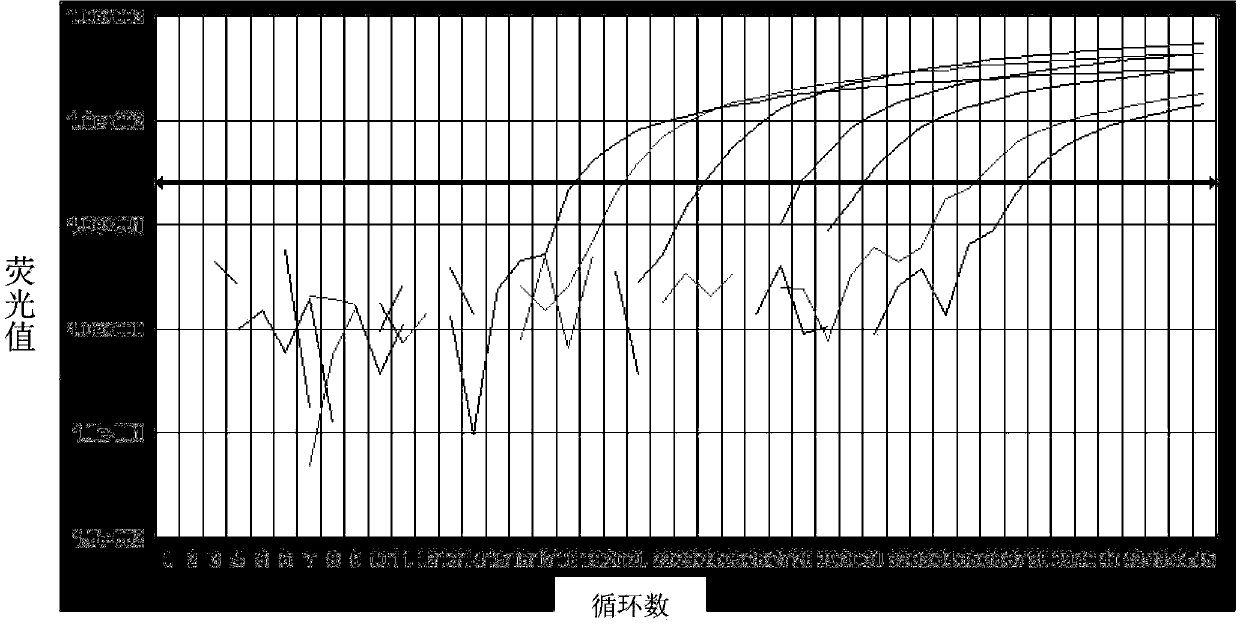
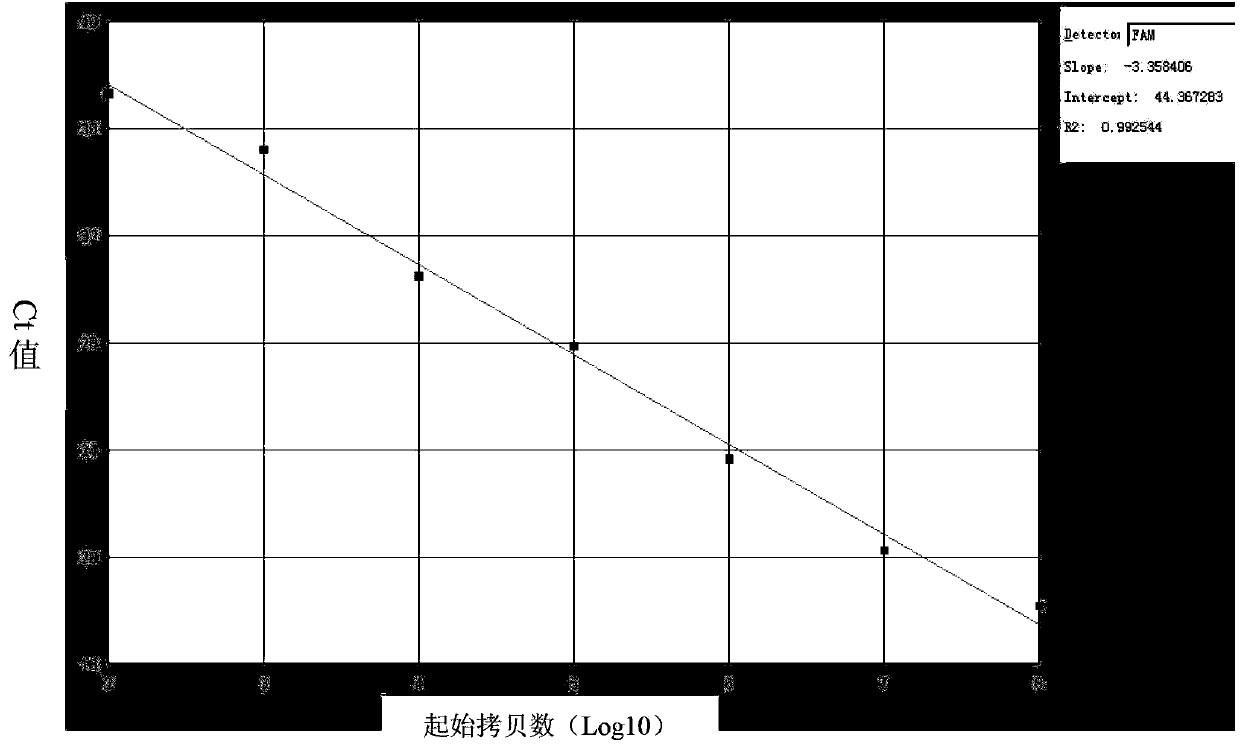
Kit for quantitatively detecting expression level of specific gene 1 mRNA (messenger Ribonucleic Acid) in human breast cancer
A breast cancer and kit technology, applied in the field of kits for quantitatively detecting the expression level of human breast cancer-specific gene 1 mRNA, can solve the problems of no positive rate detection technology, achieve good accuracy and specificity, and high detection specificity , the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, kit for quantitatively detecting human breast cancer specific gene 1 mRNA expression level
[0045] The composition of the kit and its preparation method are as follows:
[0046] 1. Red blood cell lysate
[0047] The solvent is water, the solute and its concentration in the red blood cell lysate are respectively 1.52 mol / L of ammonium chloride, 0.1 mol / L of potassium bicarbonate and 0.01 mol / L of disodium edetate. The red blood cell lysate has a pH value of 7.2 at 25°C.
[0048] 2. RNase-free water
[0049] Add diethyl pyrocarbonate (DEPC, purchased from Biobasic Company) to deionized water to a final concentration of 0.05% (volume percentage), place at 22-25°C for 10-12 hours, then autoclave at 121°C for 20 minutes , placed at 22-25°C for later use.
[0050] 3. RNA extraction solution
[0052] 4. dNTP mixture
[0053] Contains dATP, dCTP, dGTP, and dTTP, and is stored in the form of its sodium salt-water solution. At 25...
Embodiment 2
[0168] Example 2, detection of breast cancer specific gene 1 mRNA expression
[0169] The kit prepared in Example 1 was used to detect the expression level of breast cancer specific gene 1 mRNA in the specimens of the following experimental group and control group.
[0170] Experimental group: 33 cases of pathologically diagnosed breast cancer patients, of which 12 cases have been clinically diagnosed with metastasis.
[0171] Control group: 15 patients with benign breast disease and 10 healthy people.
[0172] 1. Experiment preparation
[0173] 1. Dilute the erythrocyte lysate with sterilized deionized water at a ratio of 1:9 to form the erythrocyte lysate dilution.
[0174] 2. Prepare 25mL of 75% ethanol solution.
[0175] 2. Sampling
[0176] Take 3 mL of fresh venous blood from the subject in a sterile centrifuge tube, and use EDTA as an anticoagulant (1.44 mg / mL whole blood). The sample should be used immediately after collection. If it cannot be used immediately, it...
Embodiment 3
[0218] Embodiment 3, test kit and application thereof
[0219] 1. Kit composition and preparation method
[0220] Same as described in Example 1, the difference is as follows:
[0221] 1. PCR reaction buffer: composed of tris(hydroxymethyl)aminomethane hydrochloride with a final concentration of 17.9mmol / L, magnesium chloride with a final concentration of 2.55mmol / L and potassium chloride with a final concentration of 90.0mmol / L , the solvent is water; wherein, the final concentration is the final concentration of each substance in the PCR reaction buffer.
[0222] 2. PCR reaction solution: composed of the PCR reaction buffer, Taq enzyme, dATP, dCTP, dGTP, dTTP, dUTP, uracil DNA glycosylase (UNG enzyme) and the primer pair and probe of Example 1, the rest is water;
[0223] The concentration of the PCR reaction buffer in the PCR reaction solution is 0.9 μl / μl;
[0224] The concentration of the Taq enzyme in the PCR reaction solution is 0.08U / μl;
[0225] The concentration...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com