Electrochemical transducer for mercury ion detection and manufacturing method and detection method thereof
A manufacturing method and sensor technology, applied in the field of biological analysis, can solve the problems of narrow detection range and insufficient sensitivity, and achieve the effects of high specificity, high detection sensitivity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
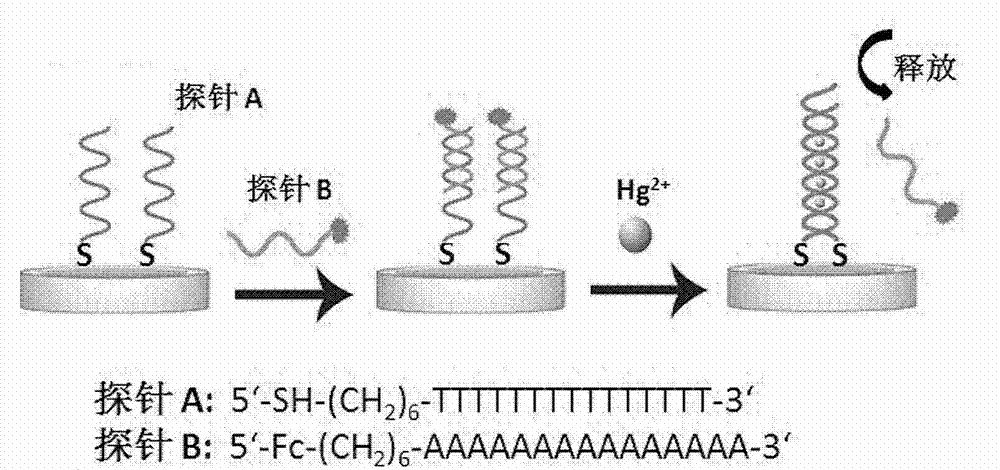
[0045] Example 1: Preparation of Hg using Probe A and Probe B 2+ Detection of electrochemical sensors (based on method A).
[0046] 1 μM Probe A (full thymine (T) oligonucleotide chain with terminal sulfhydryl modification) was reduced in 100 μM tris[2-carboxyethyl]phosphine (TCEP), 10 mM Tris, 1.0 M NaCl, pH=8.0 at room temperature 1h, soak unmodified and clean gold electrodes in it, and self-assemble overnight at room temperature. The assembled electrode was rinsed twice with 10mM Tris, 1.0M NaCl, pH = 8.0, then placed in an aqueous solution containing 1mM mercaptohexanol (MCH) to block for 30 minutes, and then washed with 10mM Tris, 1.0M NaCl, pH = 8.0 Rinse three times, dry and set aside; Dilute the ferrocene-labeled probe B (full adenine (A) oligonucleotide complementary strand) to 50nM with hybridization solution 1 / 15M PB, 0.3M NaCl, pH=7.4 , soak the gold electrode assembled with all thymine (T) oligonucleotide chains in it, react at 25°C for 3h, and then wash three t...
Embodiment 2
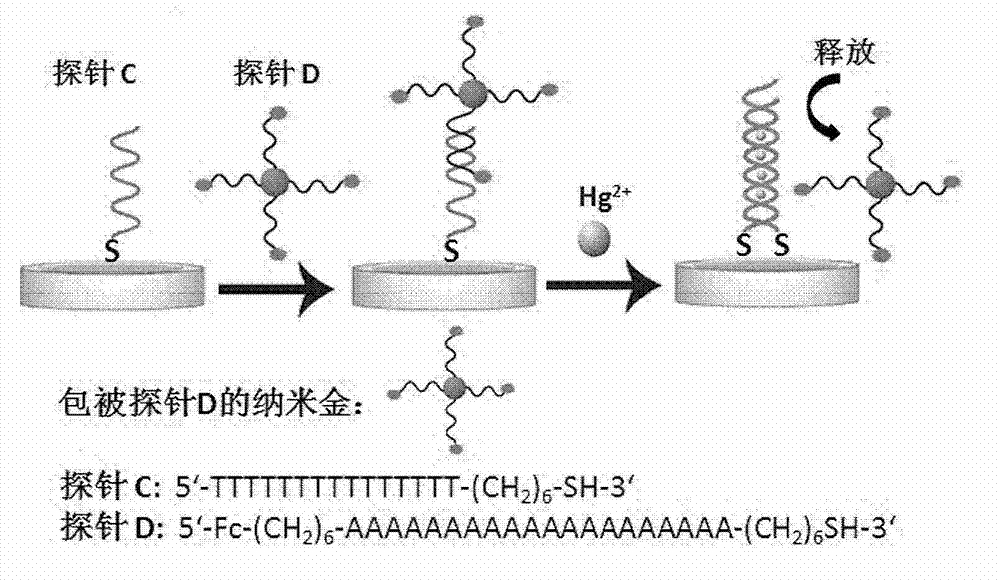
[0047] Example 2: Preparation of Hg using Probe C and Probe D 2+ Detection of electrochemical sensors (based on method B).
[0048] 1 μM Probe C (full thymine (T) oligonucleotide chain with terminal sulfhydryl modification) was reduced in 100 μM tris[2-carboxyethyl]phosphine (TCEP), 10 mM Tris, 1.0 M NaCl, pH=8.0 at room temperature 1h, soak unmodified and clean gold electrodes in it, and self-assemble overnight at room temperature. The assembled electrode was rinsed twice with 10mM Tris, 1.0M NaCl, pH = 8.0, then placed in an aqueous solution containing 1mM mercaptohexanol (MCH) to block for 30 minutes, and then washed with 10mM Tris, 1.0M NaCl, pH = 8.0 Rinse three times and dry it for later use; Dissolve the probe D (full thymine (T) oligonucleotide chain complementary to all adenine (A) oligonucleotide complementary chain) labeled ferrocene to 50 mM Dithiothreitol (DTT), 2% triethylamine (TEA) aqueous solution, room temperature, reaction 20min. Use NAP-5column to pass t...
Embodiment 3
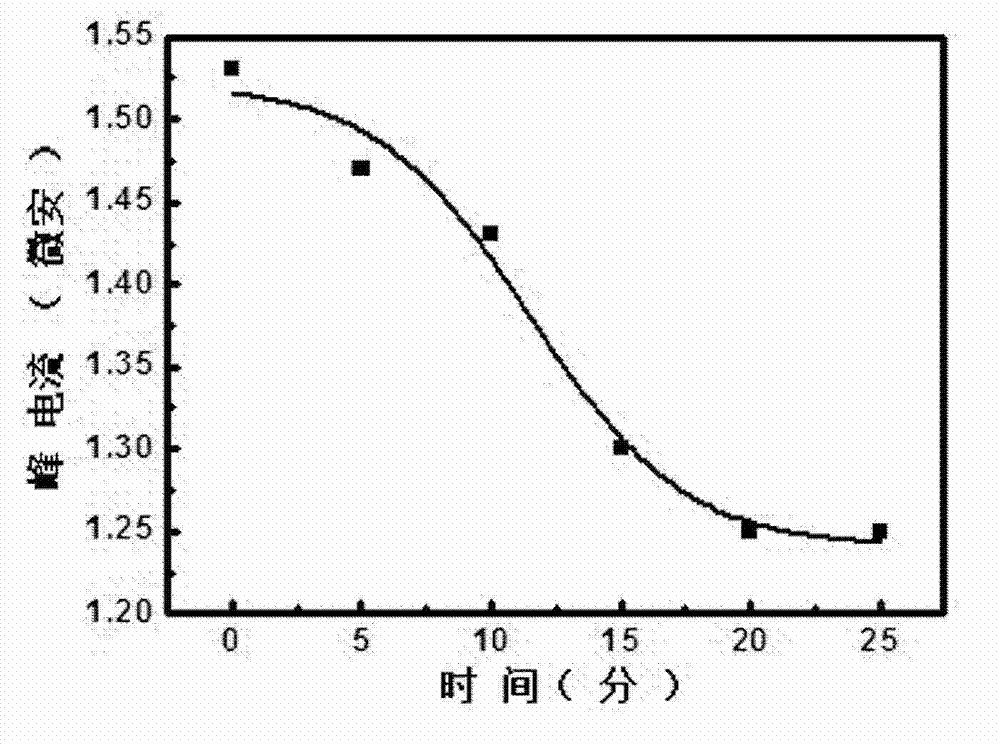
[0049] Embodiment 3: Utilize the electrochemical sensor prepared by probe A and B to investigate Hg 2+ reaction time kinetics.
[0050] The electrochemical sensors prepared with probes A and B were soaked to 1 nM Hg 2+ Soak in the buffer solution (1 / 15M PB, 0.3M NaCl, pH=7.4) at room temperature, take out the electrode every reaction for 5 minutes, wash with 1 / 15M PB, 0.1M NaCl, pH=7.4 solution for 3 times, and use a multi-channel constant Potentiometer (VMP3) to do SWV scan and analyze the results ( figure 2 ).
[0051] The results showed that Hg 2+ Inducing the release of probe B is a relatively rapid process, and 1nM Hg 2+ The reaction was basically completed within 20 min. At 25min, the SWV peak current no longer decreases.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com