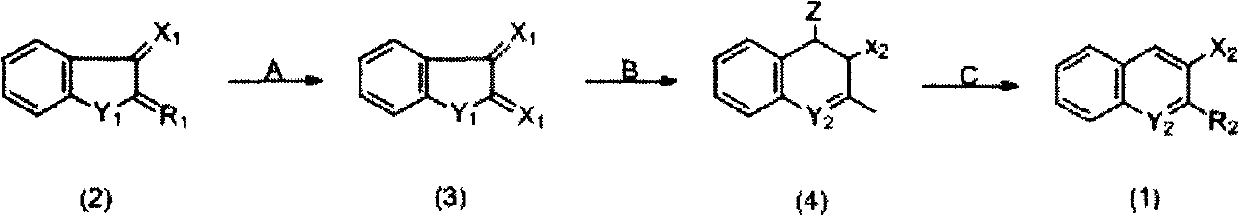
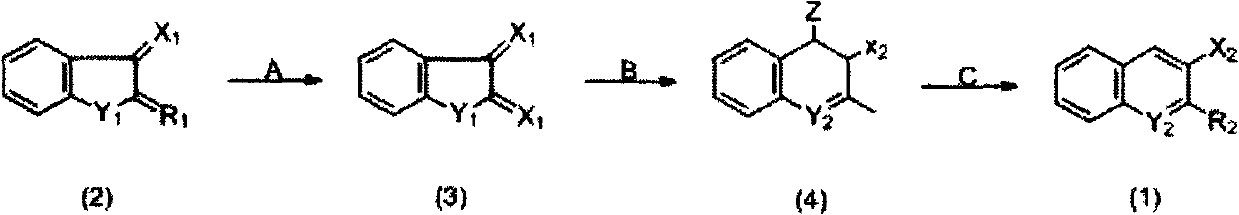
Preparation method of indanylidene compound
The technology of a compound and indanedione is applied in the field of preparation of indanedione compounds, which can solve the problems of complicated operation, environmental pollution, long reaction route and the like, and achieve the effects of simple operation steps, environmental friendliness and high product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] According to the above synthetic route, in the reaction bottle, 30g of raw material compound (2) (produced by Hebei Anping County Chemical Factory, product batch number 200910) was added into 180g 20% H 2 SO 4 Stir in the aqueous solution, and dropwise add 45g of 20% H 2 SO 4 A solution in which 30.7 g of sodium dichromate was dissolved in the solution (the molar ratio of the oxidizing agent to the raw material (2) was 0.9:1). After dropping, warm up to room temperature. Stir the reaction for 4 hours, and measure the end point of the reaction with starch-potassium iodide test paper. The reactant was filtered to obtain the intermediate compound (3) filter cake. The filter cake is directly dissolved in 400ml water without purification and contains Ca(OH) 2 In 15g of alkaline aqueous solution, the Ph value is 8-10. Heat up to 50°C, then add 23g Ca(OH) 2 , and dropwise added 17.2g of chloroacetone (B) (the molar ratio of chloroacetone to the raw material compound (...
Embodiment 2
[0024] Add 30g raw material compound (2) (source is the same as embodiment 1, hereinafter the same) in reaction flask, 46g H 2 O, 80%H 2 SO 4 66g. The reaction solution was cooled to 10°C, and Na was added dropwise 2 Cr 2 o 7 Solution 84g (Na 2 Cr 2 o 7 37.5g, H 2 O 46g) (the molar ratio of oxidant and raw material (2) is 1.1:1), the temperature is maintained at 10-12°C, after the dropwise addition is completed, the temperature is raised to 65°C for 1-2h, and the reaction end point is measured with starch-potassium iodide test paper. After the reaction, cool to room temperature and filter to obtain the filter cake of intermediate compound (3). The filter cake was directly added to 400ml of water, heated to 50°C, and adjusted to pH 8-9 with NaOH aqueous solution. Add Ca(OH) 2 26g, heat the solution to about 55°C and add 18g of chloroacetone (B) dropwise under stirring (the molar ratio of chloroacetone to raw material compound (2) is 1.7:1), raise the temperature to ...
Embodiment 3
[0026] Add raw material compound (2) 30g and 20%H in the reaction flask 2 SO 4 Stir 180g, add dropwise 45ml of aqueous solution containing 30.7g of sodium dichromate (the molar ratio of oxidizing agent and raw material (2) is 0.9:1) at 2 Afterwards, the temperature was raised to 80° C. and 16 g of chloroacetone (B) was added dropwise (the molar ratio of chloroacetone to the raw material compound (2) was 1.5:1), kept for 5 hours, and the end point of the reaction was detected by TLC. After the reaction, the temperature was lowered to 20°C, HCl was added dropwise until pH = 2, cooled, filtered, and dried to obtain 33.8g of compound (4), mp.121-124°C, yield 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com