Liver targeting molecules
A liver-targeted, molecular technology applied in the field of single variable domains of immunoglobulins (antibodies), which can solve problems such as virus resistance and side effects that hinder the development and widespread use of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0171] Example 1: Cloning and expression of human and mouse asialoglycoprotein H1 receptor subunits
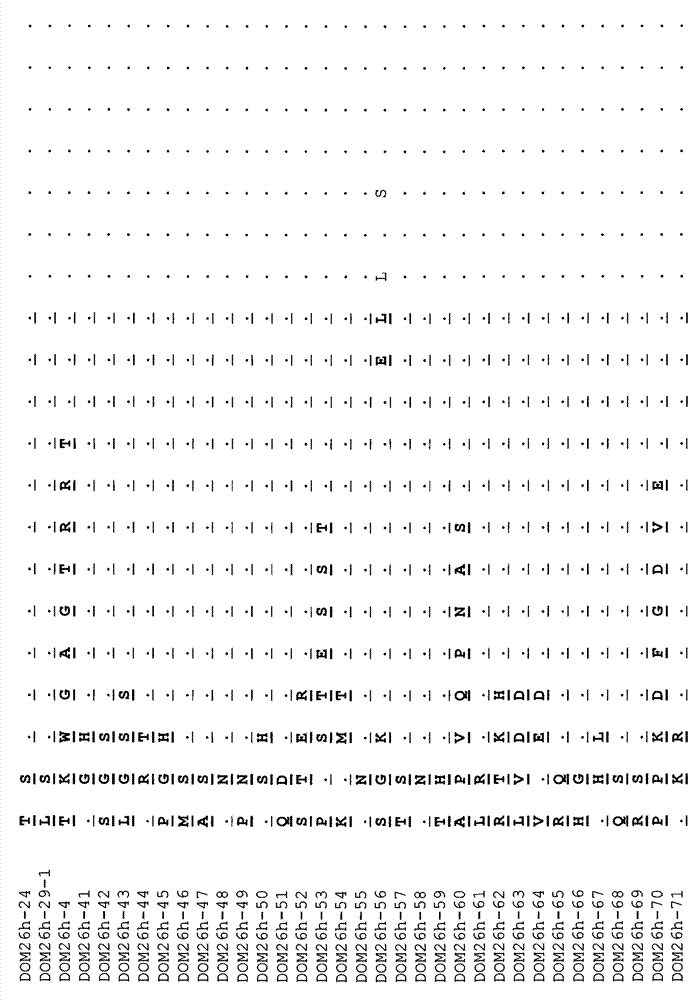
[0172] Full-length human and mouse asialoglycoprotein H1 receptor subunit (ASGPR H1) cDNAs were custom-synthesized by DNA2.0 (Mealo Park CA, USA). Using primers DLT007 and DLT008 (human) or DLT009 and DLT010 (mouse), amplify by PCR coding with N-terminal (His) 6 Tag the DNA for the extracellular domain (Q62-L291 for human, and S60-N284 for mouse). The sequences are shown in Table 1 below.
[0173] Table 1:
[0174]
[0175] The PCR fragment was inserted into the containment vector pCR-Zero Blunt (Invitrogen) by topoisomerase cloning and sequenced to obtain error-free clones using M13 forward and M13 reverse primers. Obtained by gel purification after BamHI / HindIII digestion of pCR-Zero Blunt containing the insert (His) 6 - ASGPR H1 encoding DNA and ligation of the insert into the corresponding sites in pDOM50, which is a mammalian expression vector which is a derivative...
Embodiment 2
[0192] Example 2 - Method for selecting dAbs
[0193] Domantis' 4G and 6G naive phage libraries displaying for 4G an antibody single variable domain expressed from the GAS1 leader (see WO2005 / 093074) and for 6G additionally having heat / Cold preselection (see WO04 / 101790); pool 1 contains libraries 4VH11-13 and 6VH2, pool 2 contains libraries 4VH14-16 and 6VH3, pool 3 contains libraries 4VH17-19 and 6VH4, and pool 4 contains libraries 4K and 6K. Library aliquots were of sufficient size to allow 10-fold overrepresentation of each library. Option to use passive coated and biotinylated human and mouse (His) 6 - ASGPR H1 antigen execution. Selection using passively coated antigens was performed as follows. on immunotubes (Nunc) supplemented with 5 mM Ca2 +( TBS / Ca 2+ ) in TBS, will work with 2% Marvel's TBS / Ca 2+( MTBS / Ca 2+ ) solution closed. Will work with TBS / Ca 2+ Prior to washing, store aliquots of the library in MTBS / Ca 2+ incubate. Bound phage were then eluted wi...
Embodiment 3
[0196] Example 3 - Screening for selection output of liver cell-specific dAbs
[0197] After 3 rounds of selection, the dAb genes from each library pool were subcloned from the pDOM4 phage vector into the pDOM10 soluble expression vector. pDOM4 is a derivative of the fd phage vector in which the gene III signal peptide sequence is replaced with the yeast glycolipid-anchored surface protein (GAS) signal peptide. It also contains a c-Myc tag between the leader sequence and gene III. In each case, after selection, phage DNA libraries from appropriate selection cycles were prepared using the QIAfilter midiprep kit (Qiagen), the DNA was digested with restriction enzymes Sal1 and Not1, and the enriched dAb genes were ligated into pDOM10. within the corresponding location.
[0198]The pDOM10 vector is a pUC119-based vector. Protein expression is driven by the LacZ promoter. The GAS1 leader sequence (see WO 2005 / 093074) ensures secretion of the isolated soluble dAbs into the perip...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com