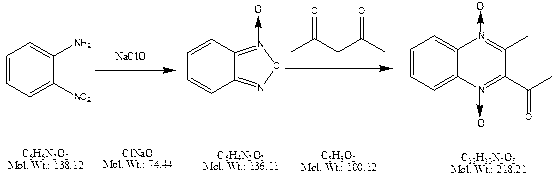
Preparation method of mequindox
A technology of acemetquine and benzofurazan, which is applied in the field of preparation of acemetquine, can solve the problems of waste of raw materials, lower product quality, and easy generation of impurities, so as to improve yield and product quality, reduce material and financial resources, Effect of reducing water content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add 54g of o-nitroaniline, 18g of lye (30%), and 320g of sodium hypochlorite (10% to 15% of available chlorine) into a 500ml three-necked flask, and keep it warm for 10 hours at a temperature of 15°C, then add 80ml of xylene, Stir for 10 minutes to completely dissolve the benzofurazan produced by the reaction, transfer it to a 500ml separating funnel and let it stand for 15 minutes, remove the lower water layer and mechanical impurities, transfer the remaining organic layer into a 250ml three-neck flask, add 0.8g potassium hydroxide , the temperature was raised to 25°C, 38.5 g of acetylacetone was added dropwise, and after the addition was completed, the reaction was incubated for 6 hours, centrifuged, washed, and dried to obtain 65.8 g of acetylmethaquine, with a yield of 77.13%.
Embodiment 2
[0041] Add 54g of o-nitroaniline, 18g of lye (30%), and 320g of sodium hypochlorite (10% to 15% of available chlorine) into a 500ml three-necked flask, and keep it warm for 10 hours at a temperature of 20°C, then add 100ml of toluene and stir The benzofurazan produced by the reaction was completely dissolved in 10 minutes, transferred to a 500ml separatory funnel and left to stand for 15 minutes, the water layer and mechanical impurities below were separated, the remaining organic layer was transferred to a 250ml three-necked flask, 2.7g of ethanolamine was added, and the temperature was raised to At 30°C, 38.5 g of acetylacetone was added dropwise. After the addition, the mixture was incubated for 10 hours, then lowered to room temperature, centrifuged, washed, and dried to obtain 67.1 g of methylquine, with a yield of 78.65%.
Embodiment 3
[0043] Add 54g of o-nitroaniline, 18g of lye (30%), and 320g of sodium hypochlorite (10% to 15% of available chlorine) into a 500ml three-neck flask, and keep it warm for 6.5 hours at a temperature of 20°C, then add 200ml of toluene and stir For 10 minutes, transfer to a 500ml separating funnel and let it stand for 15 minutes to separate the lower water layer and mechanical impurities. Transfer the remaining organic layer to a 250ml three-neck flask, add 2g of diethylamine, heat up to 65°C, and dropwise add 38.5g of acetylacetone After the addition, the reaction was incubated for 6 hours, then cooled to room temperature, centrifuged, washed, and dried to obtain 68.3 g of methquine, with a yield of 80.06%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com