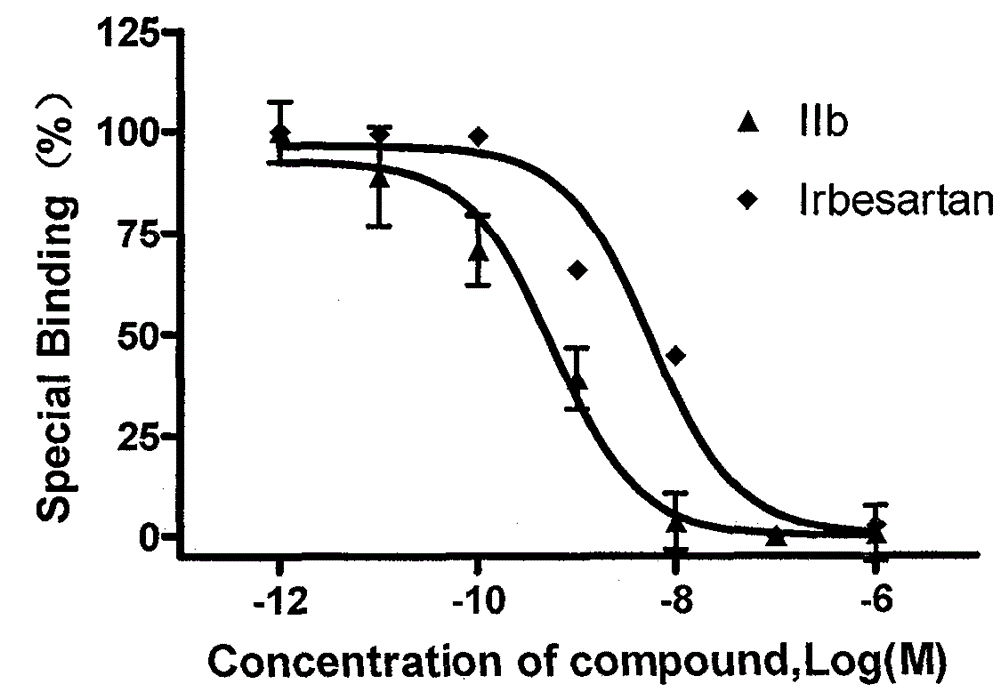
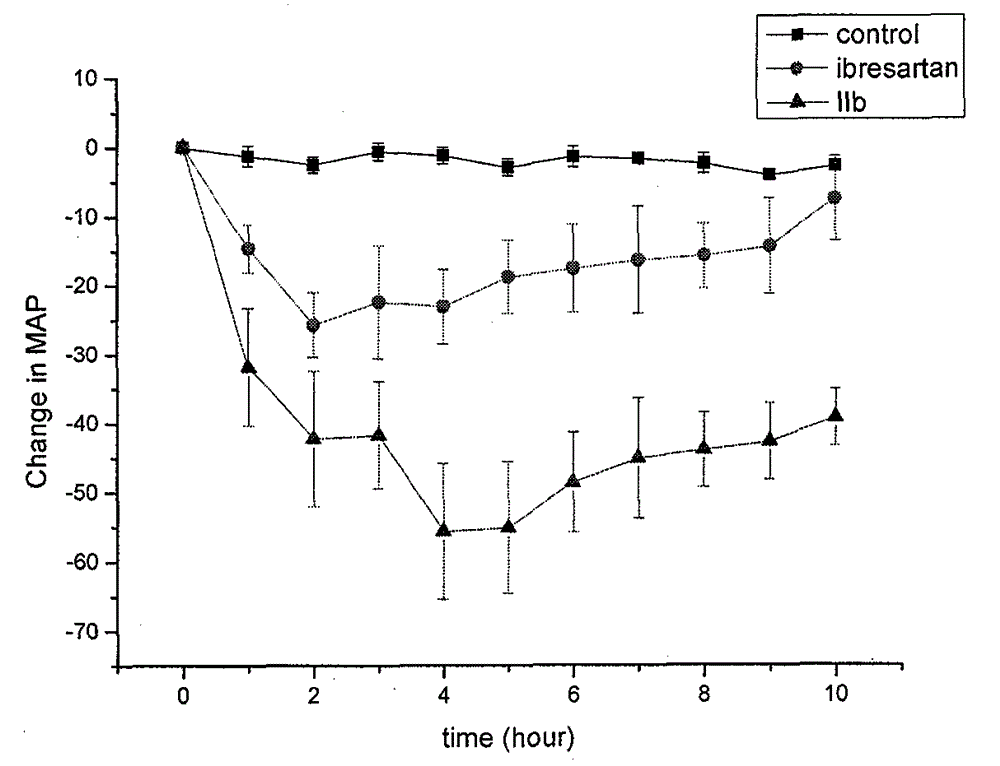
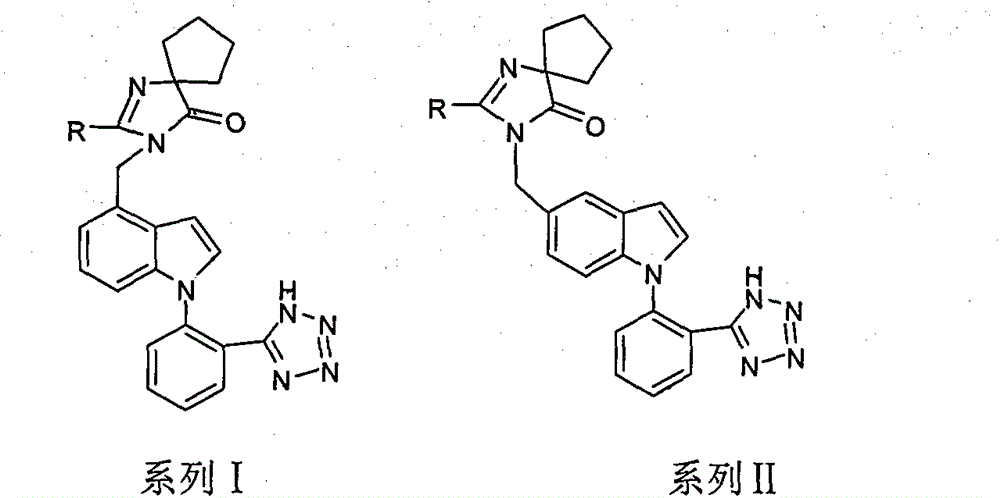
Heterospirone n-phenylindole compound, its preparation method and application
A technology of phenylindole compound and heterospiroketone, which can be applied in the field of medicinal chemistry and can solve problems such as metabolic instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The compound: 2-butyl-3-[[1-[2-(1H-tetrazol-5-yl)phenyl]-1H-indol-4-yl]methyl]-1,3-di The preparation method of azaspiro[4,4]non-1-en-4-one (compound Ib) specifically comprises the following steps:
[0025]
[0026] Step 1: Synthesis of 2-butyl-3-[(1-benzoyl-1H-indol-4-yl)methyl]-1,3-diazaspiro[4,4]non-1-ene -4-ketone (compound Ib 3 )
[0027] Butyl heterospirone (compound Ib 1 ) was prepared by referring to literature (J Med Chem, 1993, 36: 4230-4238). (4-(bromomethyl)-1H-indol-1-yl)(phenyl)methyl ketone (compound Ib 2 ) was prepared with reference to literature (J Med Chem, 1993, 36, 3371-3380). Butylheterospirone (800 mg, 3.48 mmol) and 60% NaH (293 mg, 7.33 mmol) were dissolved in 15 mL of anhydrous THF. Under nitrogen protection, stir at 50 °C for 30 min. After cooling to room temperature, 10 mL of anhydrous THF solution containing (4-(bromomethyl)-1H-indol-1-yl)(phenyl)methyl ketone (1000 mg, 3.18 mmol) was slowly added dropwise. Then heated and stirred...
Embodiment 2
[0035] 2-Propyl-3-[[1-[2-(1H-tetrazol-5-yl)phenyl]-1H-indol-4-yl]methyl]-1,3-diazaspiro The preparation method of cyclo[4,4] non-1-en-4-one (I a):
[0036] The experimental procedure is as described in Example 1, and the yield is 46.7%. 1 HNMR (400MHz, CDCl 3 )δ: 8.20 (1H, m, N-CH), 7.59-6.70 (7H, m, Ph-H), 6.54 (1H, m, CH=C), 4.91 (2H, s, N-CH 2 ), 2.25-2.22 (2H, t, J=7.2Hz, C H 2 CH 2 CH 3 ), 1.86 (8H, m, CH 2 CH 2 CH 2 CH 2 ), 1.49 (2H, m, C H 2 CH 3 ), 0.85-0.81 (3H, t, J=7.2HZ, -CH3); MS (ESI) m / z 454.5 [M+1] +
Embodiment 3
[0038] 2-Propyl-3-[[1-[2-(1H-tetrazol-5-yl)phenyl]-1H-indol-5-yl]methyl]-1,3-diazaspiro The preparation method of cyclo[4,4]non-1-en-4-one (IIa):
[0039] The experimental procedure is as described in Example 1, and the yield is 45.9%. 1 HNMR (400MHz, CDCl 3 )δ: 8.05 (1H, m, N-CH), 7.59-6.70 (7H, m, ph-H), 6.54 (1H, m, CH=C), 4.91 (2H, s, N-CH 2 ), 2.25-2.22 (2H, t, J=7.2Hz, C H 2 CH 2 CH 3 ), 1.96 (8H, m, CH 2 CH 2 CH 2 CH 2 ), 1.59 (2H, m, C H 2 CH3 ), 0.85-0.81 (3H, t, J=7.2Hz, -CH3); MS (ESI) m / z: 454.5 [M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com