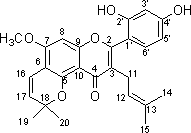
Prenylflavonoid compound and application thereof in preparation of pancrelipase inhibitor
A technology of prenyl flavonoids and compounds, applied in organic chemistry, drug combinations, medical preparations containing active ingredients, etc., can solve problems such as difficult to control intestinal symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Extract and isolate the compound of the present invention from laurel wood
[0021] (1) Extraction: After 20 kg of dried stems and branches of Artocarpus hypargyreus Hance were crushed, they were extracted by percolation with 20 times the amount of 95% ethanol, the leachate was collected, concentrated and dried to obtain 1.63 kg of extract, which was dissolved in After extracting with chloroform, the recovered solvent was concentrated to dryness to obtain 214 g of chloroform extract;
[0022] (2) Separation: 50 g of chloroform extract was subjected to silica gel column chromatography, and gradient elution was carried out with petroleum ether-acetone (10:1→6:1→3:1→1:1), and the amount of each gradient was 5000 ml. Fractions were collected; the petroleum ether-acetone 1:1 fraction was subjected to silica gel column chromatography, and eluted with chloroform-acetone 4:1 (amount of 1000 ml) to obtain the compound 5-methoxy-2,2-dimethyl -9-(3-methyl-2-butenyl)-8...
Embodiment 2
[0025] Example 2 Determination of inhibitory activity of compound (I) of the present invention on pancreatic lipase
[0026]Firstly, the substrate p-Nitrophenyl acetate (Sigma Company) was formulated with phosphate buffer solution (PBS, pH 7.4) to 1.35 M; porcine pancreatic lipase (Sigma Company) was formulated with phosphate buffer solution to 10 mg / ml; compound (I) was formulated with Phosphate buffer solution was prepared into solutions of different concentrations, and then 50 μl of enzyme solution diluted 20 times, 40 μl of substrate solution diluted 1000 times, and 10 μl of test samples of different concentrations were sequentially added to a 96-well plate, and mixed Evenly, react at 25°C for 20 minutes, and detect the absorbance of each well at 405 nm every 2 minutes;
[0027] According to the absorbance at 405 nm, the inhibitory rate (%) of the test sample to pancreatic lipase was calculated, and distilled water was used as a control, and the concentration of the inhi...
Embodiment 3
[0036] Example 3 Preparation of tablets containing the compound of the present invention (I)
[0037] Grind 20 g of compound (I) and 69 g of lactose, sieve, mix evenly, moisten with water to make a soft material, pass through a 20-mesh sieve to make wet granules, dry at 80°C, sieve the granules with a 20-mesh sieve, add starch 10 g and 1 g of magnesium stearate, fully mixed and compressed into tablets, each tablet weighs 100 mg, and the active ingredient content is 20 mg.
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com