Composition containing Cistanche deserticola or Herba Cynomorii and combination of chrysanthemum, taurine, lycopene, xanthophyll and beta-carotene
A technology of lycopene and carotene, which is applied in the direction of medical preparations containing active ingredients, drug combinations, active ingredients of hydrocarbon compounds, etc., can solve the problem of abnormal lipid metabolism in organ function, difficulty in taking it for a long time, liver and kidney and Stomach side effects and other problems, to achieve the effect of enhancing fat metabolism and conversion function, enhancing the relief of eye fatigue, and enhancing liver and kidney functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 composition of the present invention
[0042] Several compositions of the present invention can be prepared by using the materials listed in the following table as starting materials and uniformly mixing them. It should be understood that all pharmaceutically acceptable solid or liquid dosage forms such as tablets, capsules, granules, powders, ointments, pills, oral liquids and teabags can be made from the required auxiliary materials of the prior art additive type as required. Composition products.
[0043] example raw material 1 Cistanche extract 100g; Taurine 2000g; Chrysanthemum extract 2000g 2 Cistanche extract 900g; Taurine 200g; Chrysanthemum extract 200g 3 Cistanche extract 400g; Taurine 1000g; Chrysanthemum extract 1000g 4 Cistanche deserticola extract 100g; Lutein 30g 5 Cistanche deserticola extract 900g; Lutein 6g 6 Cistanche deserticola extract 400g; Lutein 15g 7...
Embodiment 2
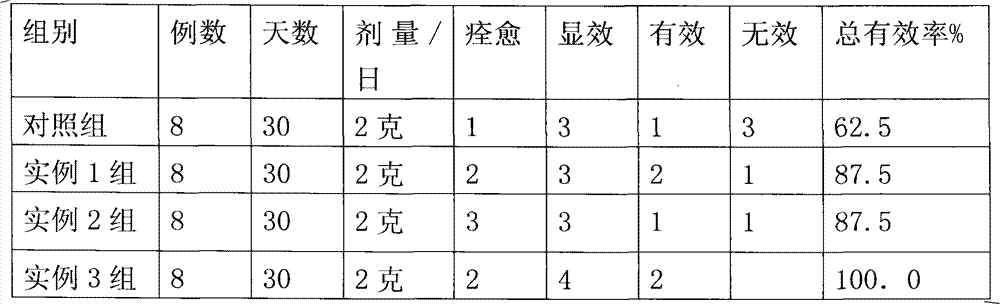
[0045] Example 2 Experiment of the composition of the present invention for alleviating eye fatigue
[0046] Clinical data: 32 patients were selected, 20 males, 12 females, 9 cases of 18 years old, 16 cases of 35 years old, 7 cases of 46-55 years old; occupation: 7 cases of teachers, 16 cases of students, 6 cases of crash, 9 cases of farmers ; 20 cases of myopia, 6 cases of hyperopia, 6 cases of astigmatism; 32 cases of normal intraocular pressure, accompanied by mild dizziness, blurred vision, weak and unbalanced eye muscle strength; Method: the above 32 patients were randomly divided into 4 groups, respectively 24 cases of the present invention group, divided into 3 groups, each group was given the composition prepared by the examples 1, 2, and 3 of the present invention, and the dosage was 2 grams per person per day. Take twice; Control group 8 examples, are the composition of chrysanthemum extract and taurine, ratio and preparation are with the ratio and the preparation me...
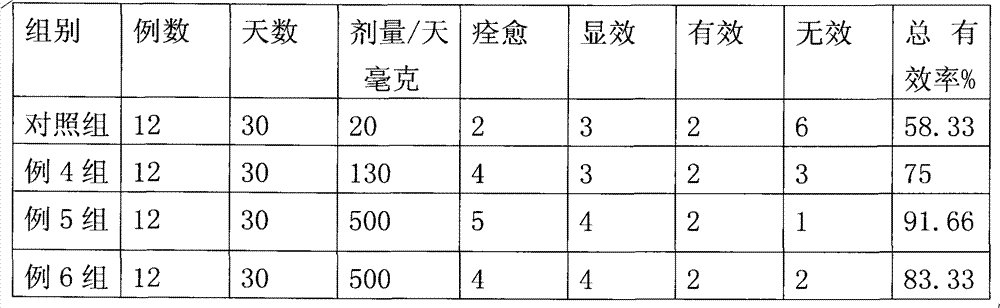
Embodiment 3
[0050] Embodiment 3 Composition of the present invention improves eyesight effect experiment
[0051] Clinical data: 48 patients were selected, 29 males, 58 eyes, 19 females, 36 eyes, age 34-50 years old, occupation: 23 teachers, 17 students, 8 farmers; 20 cases of myopia, 15 cases of hyperopia 13 cases of astigmatism; 48 cases of normal intraocular pressure, the statistical data are logarithmic vision chart and judgment standard, the course of disease is 2 years for the elder, and 6 days for the short one, and the logarithmic chart of all cases is ≤2 lines. Method: above 48 routine patients are divided into 4 groups at random, are respectively 36 cases of the present invention group, divide 3 groups, every group of 12 cases, give respectively the composition prepared in the present invention example 4,5,6, dosage is that example 4 is 130 mg / day per person, and 500 mg / day per person in Example 5 and Group 6, taking twice; 12 cases in the control group were the lutein group alo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com