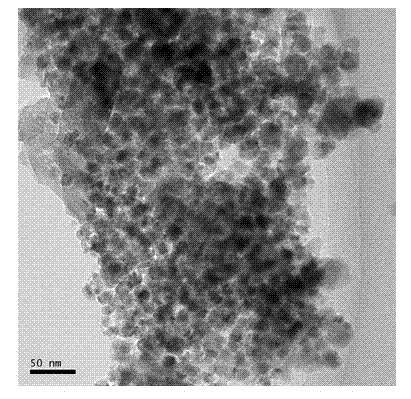
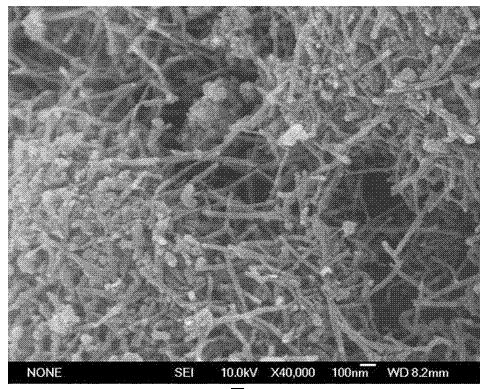
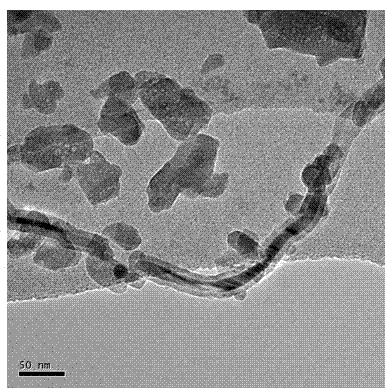
Preparation method of magnetically targeted localization magnetic drug carrier
A magnetic targeting and drug technology, which can be used in drug combinations, pharmaceutical formulations, medical preparations with inactive ingredients, etc., can solve the problems of large toxic and side effects, easy agglomeration, poor magnetic targeting ability and stability, etc. Achieve the effect of good saturation magnetization and high coercivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The first step, preparation of iron-nanometer hydroxyapatite catalyst
[0044] According to the ratio of ferric nitrate nonahydrate: hydroxyapatite=0.03:1 by mass ratio, after weighing the required amount of ferric nitrate nonahydrate and the hydroxyapatite particles of 30nm and mixing, adopt a planetary ball mill with 1000r / min Speed ball milling for 2 hours to uniformly mix ferric nitrate nonahydrate and nano-hydroxyapatite, place the mixture in a quartz ark, then place the quartz ark in a box-type resistance furnace, and then raise the temperature of the box-type resistance furnace to 300 ℃, calcined for 2 hours, and then cooled to room temperature with the furnace to obtain the iron oxide-nanometer hydroxyapatite mixture. The quartz ark containing the mixture was placed in the constant temperature zone of the horizontal tube furnace, and the Pass hydrogen into the furnace, raise the temperature to 600°C and keep it for 2 hours to reduce the iron oxide-nano-hydroxy...
Embodiment 2
[0059] The first step, preparation of iron-nanometer hydroxyapatite catalyst
[0060] According to the ratio of ferric nitrate nonahydrate: hydroxyapatite=0.76:1 by mass ratio, after weighing the required amount of ferric nitrate nonahydrate and the hydroxyapatite particles of 50nm and mixing, adopt planetary ball mill with 1200r / min Speed ball milling for 4 hours to uniformly mix ferric nitrate nonahydrate and nano-hydroxyapatite, place the mixture in a quartz ark, then place the quartz ark in a box-type resistance furnace, and then raise the temperature of the box-type resistance furnace to 400 ℃, calcined for 3 hours, and then cooled to room temperature with the furnace to obtain a mixture of iron oxide-nanometer hydroxyapatite. The quartz ark containing the mixture was placed in the constant temperature zone of the horizontal tube furnace, and the Pass hydrogen into the furnace, raise the temperature to 700°C and keep it for 3 hours to reduce the iron oxide-nanometer hyd...
Embodiment 3
[0070] The first step, preparation of iron-nanometer hydroxyapatite catalyst
[0071]Ferric nitrate nonahydrate by mass ratio: the ratio of hydroxyapatite=0.4:1, after weighing the required amount of ferric nitrate nonahydrate and the hydroxyapatite particle of 40nm and mixing, adopt planetary ball mill with 1100r / min Speed ball milling for 3 hours to uniformly mix ferric nitrate nonahydrate and nano-hydroxyapatite, place the mixture in a quartz ark, then place the quartz ark in a box-type resistance furnace, and then raise the temperature of the box-type resistance furnace to 350 ℃, calcined for 2.5h, and then cooled to room temperature with the furnace to obtain the iron oxide-nanometer hydroxyapatite mixture. Hydrogen gas was introduced into the tube furnace, the temperature was raised to 650°C and kept for 2.5 hours to reduce the iron oxide-nanometer hydroxyapatite mixture, and then the tube furnace was cooled to room temperature under a hydrogen atmosphere at a flow rat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com