Zaleplon double-release capsule and preparation method thereof
A zaleplon, double-release technology, applied in the field of zaleplon double-release capsule preparation and its preparation, can solve the problem of high blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
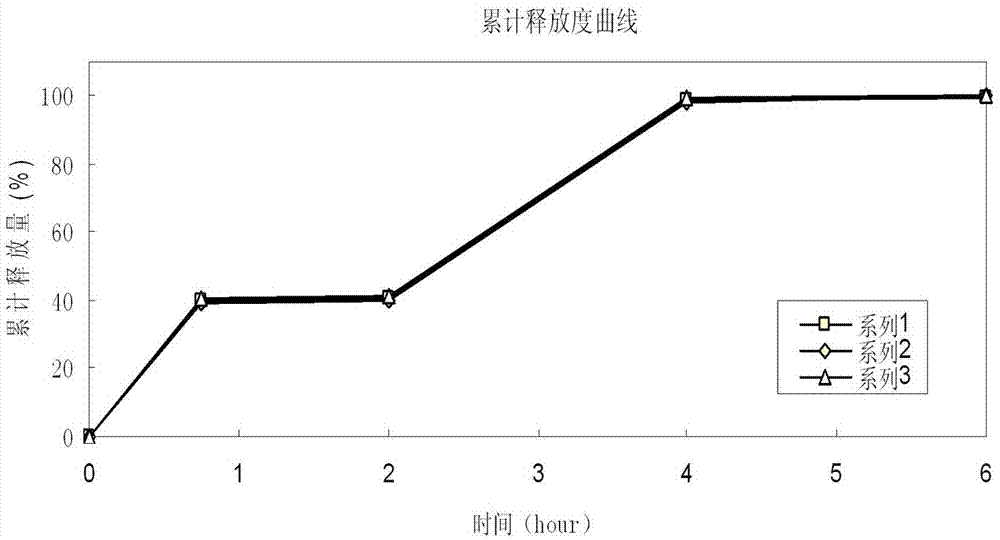
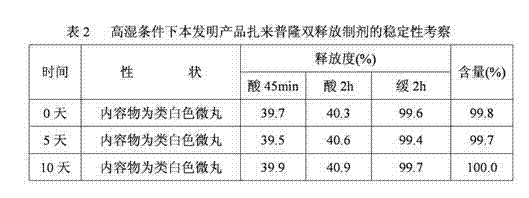
[0088] Zaleplon double-release capsules of the present invention comprise quick-release pellets and delayed-release pellets, and the weight percentages of the quick-release pellets and delayed-release pellets are respectively 40% and 60%, i.e. immediate release The mass ratio between the pellets and the delayed-release pellets is 2:3;
[0089] With respect to the immediate-release part in the zaleplon double-release capsules, the quick-release pellets are made up of the following raw materials in percentage by weight: zaleplon 16.1%, sucrose pellets 36.6%, filler mannitol 14.1% , binder hypromellose 8.1%, disintegrant A crospovidone 2.0%, isolation layer film-forming material hypromellose 13.9%, disintegrant B crospovidone 6.9% and Solvent A sodium lauryl sulfate 2.3%;
[0090] Relative to the delayed-release part in the zaleplon double-release capsule, the delayed-release pellets are made up of the following raw materials in percentage by weight: zaleplon 14.3%, sucrose pell...
Embodiment 2
[0092] The preparation method of the above-mentioned embodiment 1 zaleplon double-release capsules, its detailed steps are as follows:
[0093] Firstly, the drug-loaded pellet cores are prepared according to the drug formula, and the immediate-release pellets are prepared from the drug-loaded pellet cores, and then the delayed-release pellets are prepared from the immediate-release pellets, and finally the prepared immediate-release pellets and delayed-release pellets are respectively filled into capsules to obtain Zhalai Pron double release capsules;
[0094] (1) Preparation of drug-loaded pellet core:
[0095] The composition of the drug-loaded pill core: Zaleplon 92.4g, sucrose pellets (30-40 mesh) 210g, filler mannitol 80.85g, binder hypromellose 46.2g, disintegrant A cross-linked polymer Vitone 11.55g, 10% ethanol aqueous solution 924g (the ethanol aqueous solution is used as a solvent, and the solvent evaporates after this process);
[0096] Preparation process: first,...
Embodiment 3
[0110] Zaleplon double-release capsules of the present invention comprise quick-release pellets and delayed-release pellets, and the weight percentages of the quick-release pellets and delayed-release pellets are respectively 40% and 60%, i.e. immediate release The mass ratio between the pellets and the delayed-release pellets is 2:3;
[0111]With respect to the immediate-release part in the zaleplon double-release capsules, the quick-release pellets are made up of the following raw materials in percentage by weight: zaleplon 19.1%, sucrose pellets 43.3%, filler mannitol 16.7% , binder hypromellose 9.5%, disintegrant A crospovidone 2.4%, isolation layer film-forming material Opadry 9.0%;
[0112] Relative to the delayed-release part in the zaleplon double-release capsule, the delayed-release pellets are made up of the following raw materials in percentage by weight: zaleplon 14.7%, sucrose pellets 33.3%, filler mannitol 12.8% , binder hypromellose 7.3%, disintegrant A crospov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com