Compound sulfamethoxazole dry suspension and preparation method thereof
A technology of dried sulfamethoxazole and methoxazole, applied in the field of medicine, can solve the problems of affecting the compliance of patients, inconvenient to take, very bitter, etc., and achieve the effect of shortening the residual time of bitter taste, not affecting the taste, and taking it smoothly.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
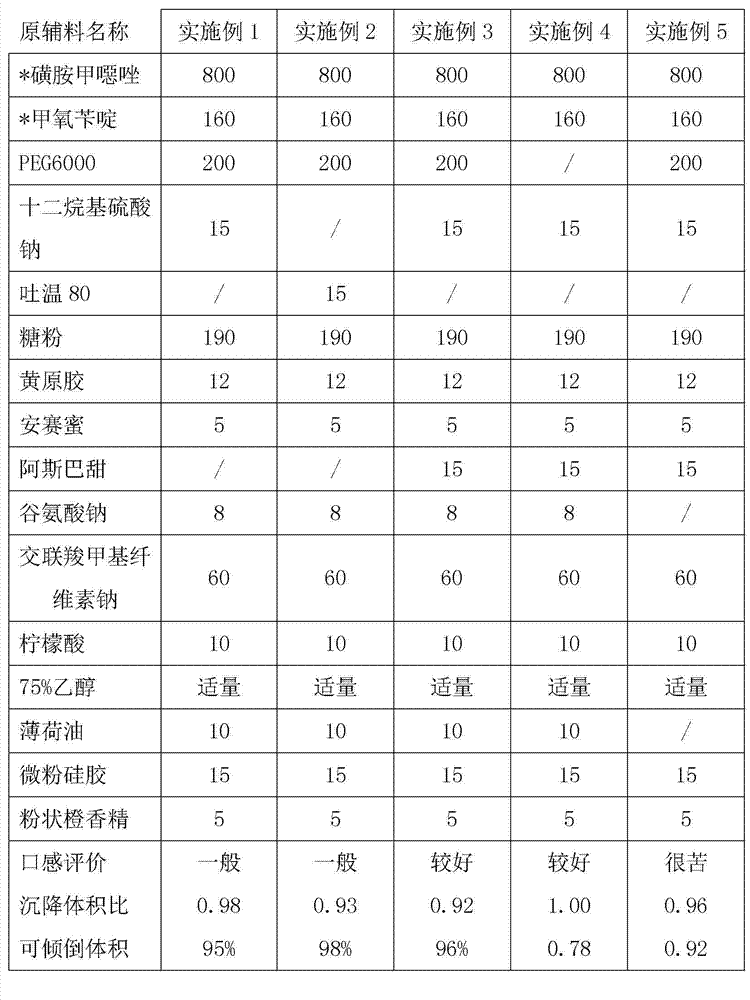
[0017] Embodiment 1: compound sulfamethoxazole dry suspension, the parts by weight of its constituent raw materials are: sulfamethoxazole 800, trimethoprim 160, PEG6000 200, sodium lauryl sulfate 15, powdered sugar 190, yellow Raw gum 12, acesulfame potassium 5, aspartame 15, sodium glutamate 8, croscarmellose sodium 60, citric acid 10, peppermint oil 10, micronized silica gel 15, powdered orange essence 5 and 75 Appropriate amount of % ethanol.
[0018] The preparation method of compound sulfamethoxazole dry suspension comprises the following steps:
[0019] (1) Weigh each raw material according to the weight of the constituent raw materials. First, put PEG6000 into a large beaker and heat it in a water bath at 90°C until PEG6000 completely changes from solid to clear liquid. Then add trimethoprim into the liquid and stir evenly. After 10 minutes, take it out, pour the mixed solution into a stainless steel plate, keep the thickness 2-3mm, place it at room temperature for 40-...
Embodiment 2
[0022] Embodiment 2: compound sulfamethoxazole dry suspension, the weight parts of its composition raw material are: sulfamethoxazole 800, trimethoprim 160, PEG6000 200, Tween 80 15, sugar powder 190, xanthan gum 12 , Acesulfame K 5, Aspartame 15, Sodium Glutamate 8, Croscarmellose Sodium 60, Citric Acid 10, Peppermint Oil 10, Micropowder Silica Gel 15, Powdered Orange Flavor 5 and 75% Ethanol .
[0023] Preparation process: mix Tween 80 and citric acid evenly, and the rest of the process is the same as in Example 1.
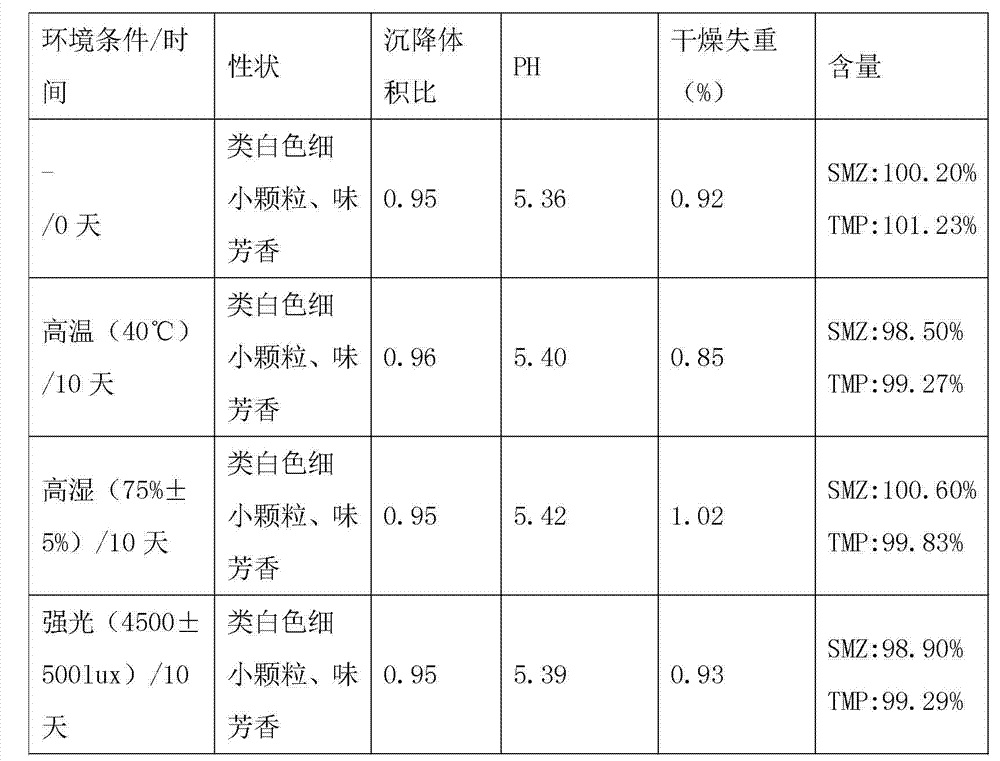
[0024] The formula of compound recipe sulfamethoxazole dry suspension of the present invention is summarized in table 1, wherein is formed evaluation group by 10 adults, the mouthfeel of compound recipe sulfamethoxazole dry suspension, is evaluated, as shown in table 1 The evaluation conclusion is that at least 7 people are under the condition of this opinion and then this evaluation conclusion is used as the evaluation conclusion of the mouthfeel of the medici...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com