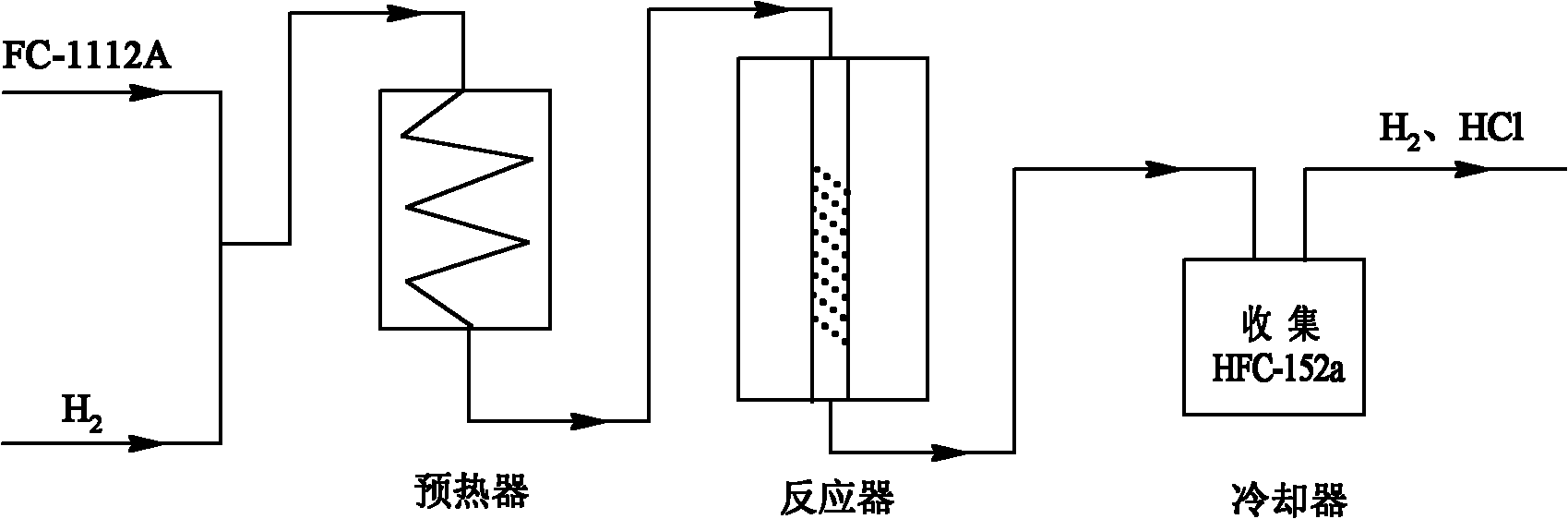
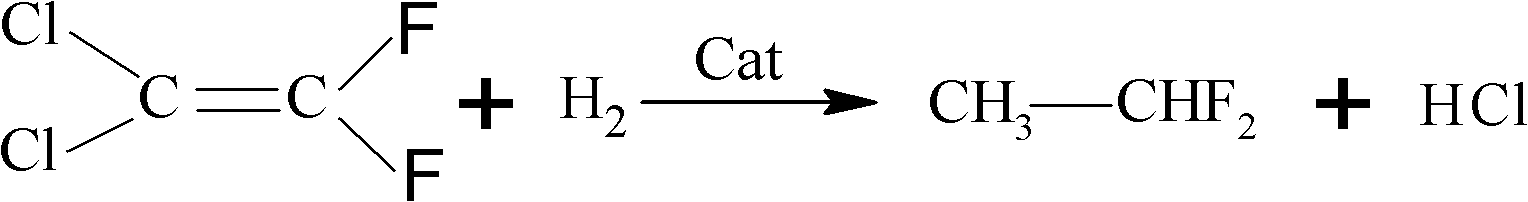
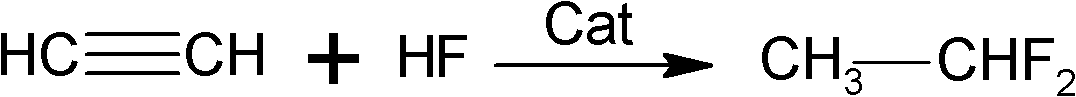Preparation method of 1,1-difluoroethane
A technology of difluoroethane and difluorodichloroethylene, applied in the field 1, can solve the problems of low utilization rate of raw materials, influence on product yield, short reaction cycle, etc., and achieve simple preparation method, easy operation, and few side reactions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: Pd / AC-300 catalyst (sample A)
[0043] Grind the activated carbon and screen out 10-20 mesh as a carrier, weigh 100g of activated carbon, put it into 300ml of 10% hydrochloric acid solution, stir and reflux for 6 hours in a water bath at a temperature of 80°C, take it out, and wash the acid with distilled water The final activated carbon is washed to neutrality, and then dried at 100°C for use;
[0044] Weigh 0.13g PdCl 2 , dissolved in 2ml of concentrated hydrochloric acid, added 15g of distilled water to make PdCl 2 After fully dissolving, add 15g of acid-washed activated carbon to the above solution, impregnate for 24 hours, dry at 100°C, then roast at 300°C for 4 hours in an air atmosphere, cool and set aside, record as: 0.5% Pd / AC-300.
Embodiment 2
[0045] Embodiment 2: Pd-Ni / AC-300 catalyst (sample B)
[0046] Weigh 1.21g NiCl 2 ·6H 2 O, dissolved in 15g of distilled water, after fully dissolving, add 15g of activated carbon treated in Example 1, impregnate for 24h, dry at 100°C, then roast at 400°C for 4h in an air atmosphere, cool and set aside;
[0047] Weigh 0.09g PdCl 2 , dissolved in 2ml of concentrated hydrochloric acid, added 10g of distilled water to make PdCl 2 After fully dissolving, add 10g of activated carbon containing auxiliary agent Ni after the above treatment, impregnate for 24h, dry at 100°C, then roast at 300°C for 4h in air atmosphere, after cooling, record as: 0.5%Pd-2% Ni / AC-300.
Embodiment 3
[0048] Embodiment 3: Pd-Ni / AC-300 catalyst (sample C)
[0049] As in the method of Example 2, PdCl will be weighed 2 The amount of the catalyst was changed to 0.05g, and the rest of the preparation conditions were unchanged, and the prepared catalyst was recorded as: 0.3%Pd-2%Ni / AC-300.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com