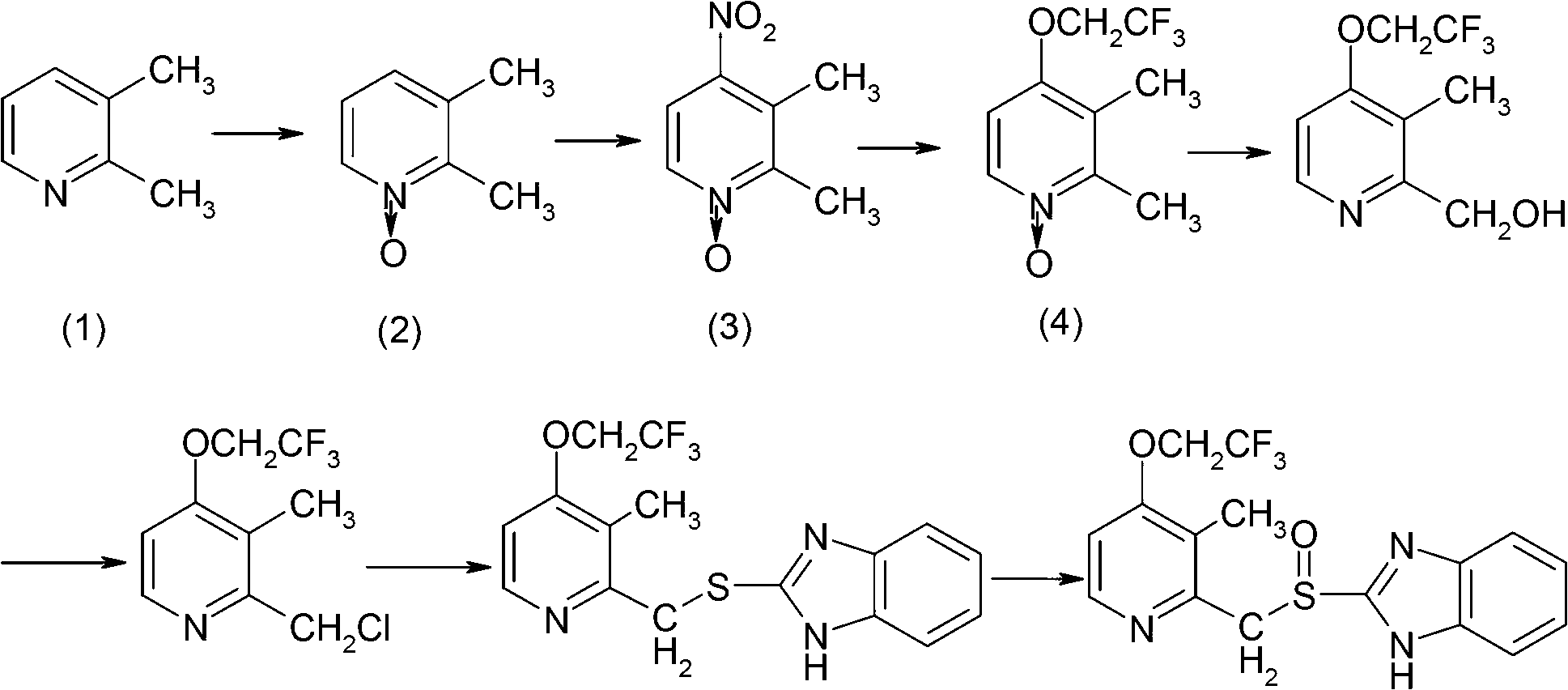
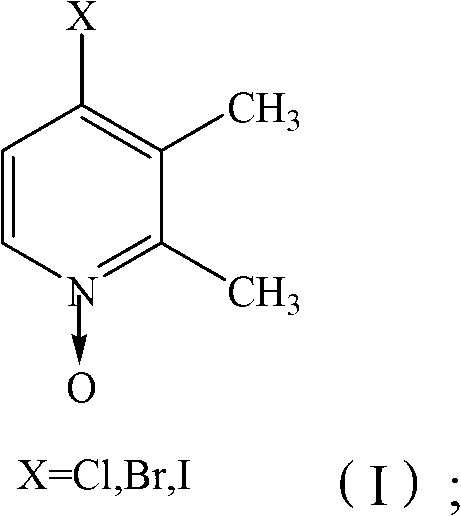
Preparation method of lansoprazole intermediate
A technology for lansoprazole and intermediates, applied in the field of preparation of lansoprazole intermediates, can solve problems such as low product yield, large purification difficulty, and increased product cost, and achieve high yield and good industrial application , The effect of safety improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The invention provides a kind of preparation method of Lansoprazole intermediate, comprises the following steps:
[0025] a) After mixing trifluoroethanol, 2,3-dimethyl-4-halopyridine-N-oxide and inorganic basic compound, carry out reflux reaction to obtain 2,3-dimethyl-4-trifluoroethane Oxypyridine-N-oxide.
[0026] In the present invention, 2,3-dimethyl-4-halopyridine-N-oxide and trifluoroethanol are used as raw materials, and reflux reaction is carried out in the presence of inorganic basic compounds to obtain 2,3-dimethyl-4-tris Fluoroethoxypyridine-N-oxide can directly carry out the preparation of lansoprazole in the next step without complicated post-treatments such as column chromatography purification and catalyst recovery. Wherein, trifluoroethanol is not only used as a reaction raw material, but also as a reaction solvent, which avoids the introduction of other solvents, and does not require fractional distillation to recover the complicated post-treatment of...
Embodiment 1
[0057] Dissolve 2,3-dimethyl-4-nitropyridine-N-oxide 48.3g (0.287mol) in 100mL ethanol to obtain 2,3-dimethyl-4-nitropyridine-N-oxide Ethanol solution, start stirring, add 500mL 25% hydrochloric acid ethanol solution to it and reflux for 3 hours to obtain a mixture of 2,3-dimethyl-4-chloropyridine-N-oxide, and then evaporate the mixture under normal pressure ethanol in the ethanol-free 2,3-dimethyl-4-chloropyridine-N-oxide mixture, to the ethanol-free 2,3-dimethyl-4-chloropyridine-N-oxide mixture After adding 200mL of water, extract with 300mL dichloromethane three times, collect the organic phase and distill off the dichloromethane in the organic phase to obtain pure 2,3-dimethyl-4-chloropyridine-N-oxide 43g, yield 95%. The product is a white solid with a melting point of 103°C~105°C. 1 H NMR (CDCl 3 , 600MHz) δ: 2.39 (s, 3H, Me), 2.55 (s, 3H, Me), 7.13 (d, 1H, Py-H), 8.08 (d, 1H, Py-H).
[0058] 150mL trifluoroethanol and 60g K 2 CO 3 (0.415moL) was mixed in a 100mL fo...
Embodiment 2
[0060] Dissolve 2,3-dimethyl-4-nitropyridine-N-oxide 48.3g (0.287mol) in 100mL ethanol to obtain 2,3-dimethyl-4-nitropyridine-N-oxide ethanol solution, start stirring, add 500mL 25% hydrobromic acid ethanol solution to it and reflux for 3 hours to obtain a mixture of 2,3-dimethyl-4-bromopyridine-N-oxide, and then evaporate it under normal pressure Ethanol in the mixture gives ethanol-free 2,3-dimethyl-4-bromopyridine-N-oxide mixture, to ethanol-free 2,3-dimethyl-4-bromopyridine-N-oxide After adding 200mL of water to the mixture, extract with 300mL dichloromethane in 3 times, collect the organic phase and distill off the dichloromethane in the organic phase to obtain 2,3-dimethyl-4-bromopyridine-N-oxide pure Product 54g, yield 93%. The product is a white solid with a melting point of 97.6°C~99.5°C.
[0061] 150mL trifluoroethanol and 45g K 2 CO 3 (0.324moL) was mixed in a 100mL four-necked flask, and the stirring was started, and 50g of 2,3-dimethyl-4-bromopyridine-N-oxide ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com