Use of indanthrene compounds in organic photovoltaics
A technology of indanthrene and compounds, applied in the field of organic solar cells, can solve problems such as limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
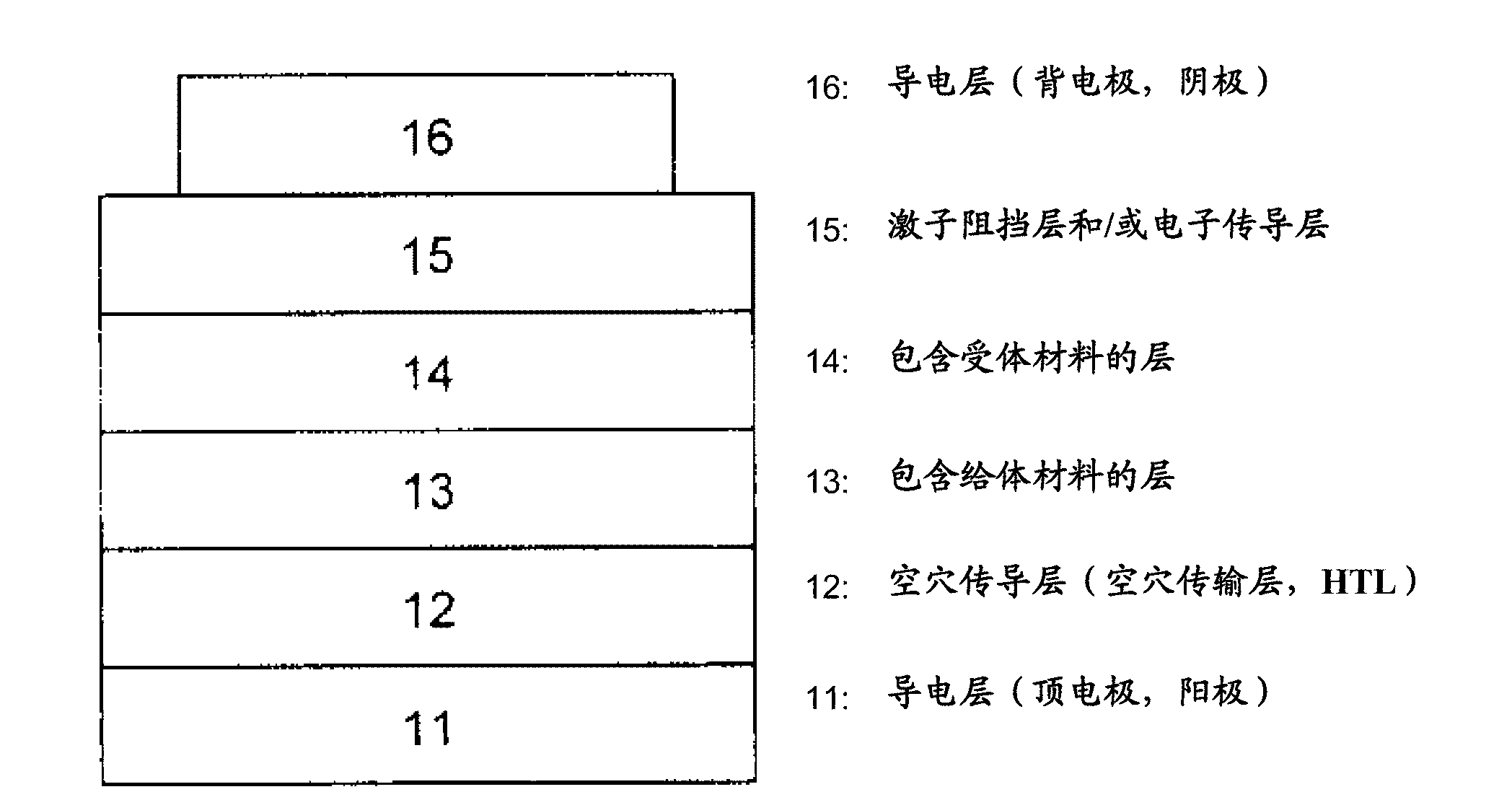
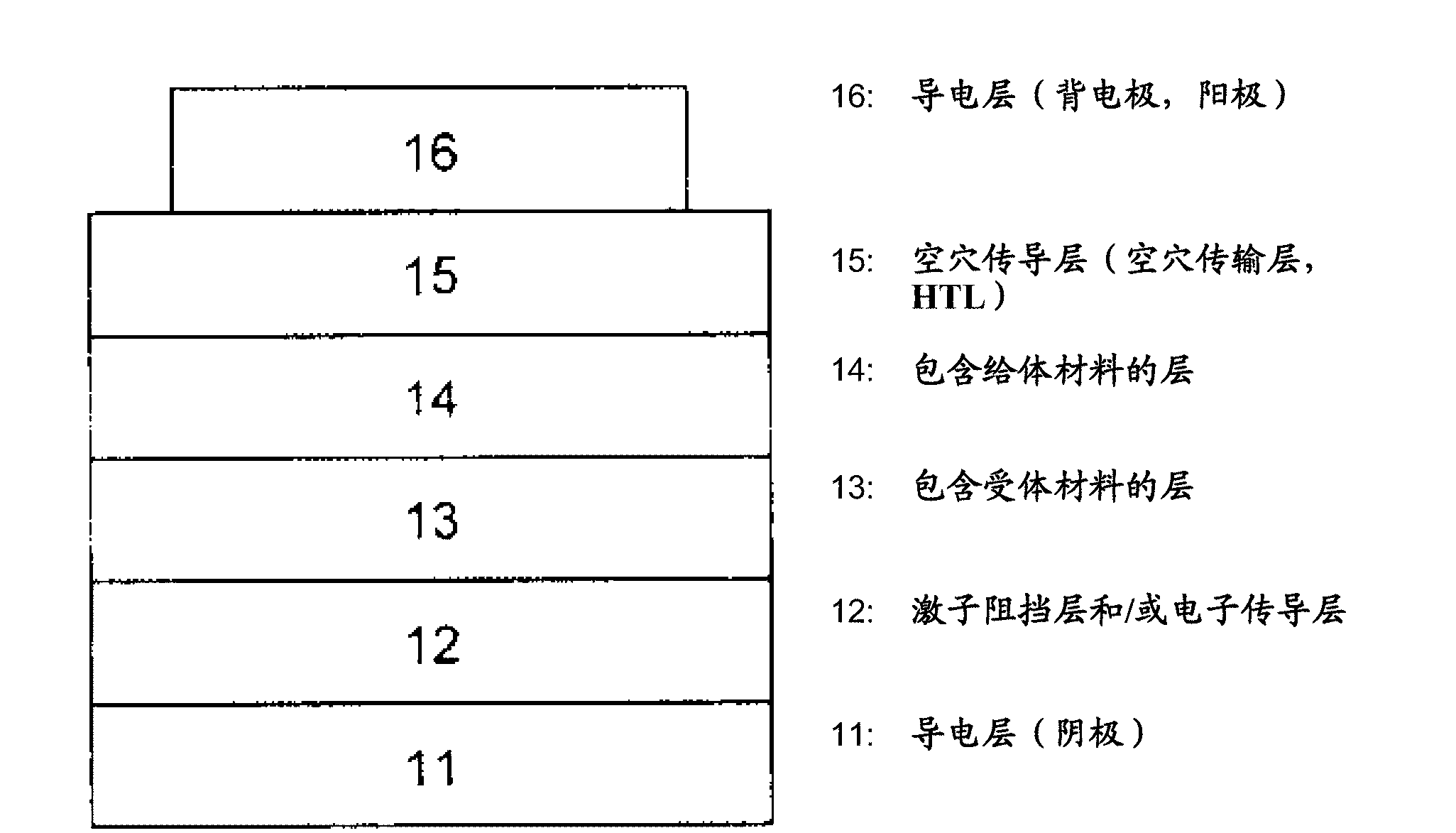
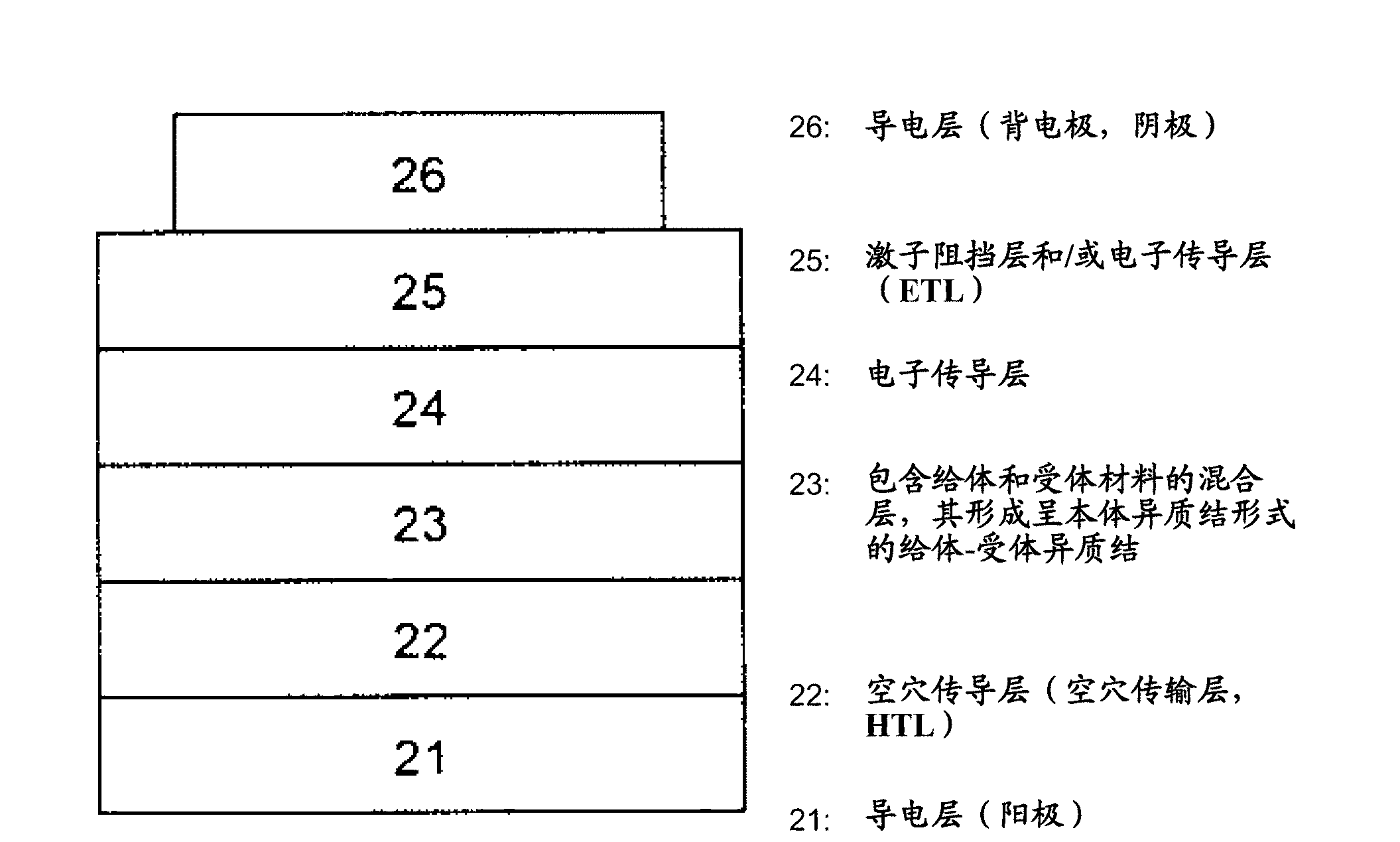
[0221] The preparation of mixed planar-hybrid heterojunctions is described in Adv. Mater. 17, 66-70 (2005). In this structure, a mixed heterojunction layer formed by simultaneous evaporation of acceptor and donor materials exists between homogeneous donor and acceptor materials.
[0222] In a particular embodiment of the invention, the donor-acceptor heterojunction is in the form of a gradient bulk heterojunction. In a mixed layer composed of donor and acceptor materials, the donor-acceptor ratio changes gradually. The gradient form can be stepped ( Image 6 (a)) or linear. exist Image 6 In (a), layer 01 is composed of 100% donor material, layer 02 has a donor / acceptor ratio > 1, layer 03 has a donor / acceptor ratio = 1, and layer 04 has a donor / acceptor ratio of Ratio Image 6 In (b), layer 01 consists of 100% donor material, layer 02 has a reduced donor / acceptor ratio, i.e. the proportion of donor material decreases linearly in the direction of layer 03, and layer 03 con...
Embodiment 1
[0264] Example 1: Deuterated Indanthrene Blue
[0265]
[0266] 3.0g of indanthrene blue in 30mol D at room temperature 2 SO 4 Stirring for 20 hours. The solution was then added to 100ml D 2 O to precipitate the product, filter and wash with D 2 O wash to neutral. This gave 2.98 g of product. The product was redissolved in 30ml D 2 SO 4 and stirred at room temperature for 20 hours. Then add 50ml D dropwise 2 O, the resulting precipitate was filtered off and the residue was washed with D 2 O wash and dry. This gave 2.9 g of deuterated product.
[0267] To fabricate solar cells, 2.0 g of this material was subjected to gradient sublimation three times at 375°C / 325°C / 250°C. This gave 829 mg of blue product.
Embodiment 2
[0268] Embodiment 2: N, N'-diphenylindanthrene
[0269]
[0270] 20.0g (45.2mmol) indanthrene blue, 28.5g (182mmol) bromobenzene, 19.25g (182mmol) sodium carbonate, 0.2g (3.18mmol) copper iodide (I) and 0.34g (1.8mmol) copper acetate The mixture of (I) was heated in 50 ml of nitrobenzene for 18 hours. The reaction mixture was cooled and filtered with suction through a filter filled with silica gel. The product was eluted with a mixture of acetone and dichloromethane. The product thus obtained is purified again by chromatography using cyclohexane / ethyl acetate (2:1). This afforded 1.8 g (7%) of a blue solid.
[0271] 630 mg of this product was subjected to gradient sublimation at 265°C / 200°C / 150°C. This yielded 160 mg of blue material, which was used for the production of solar cells.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Layer thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com