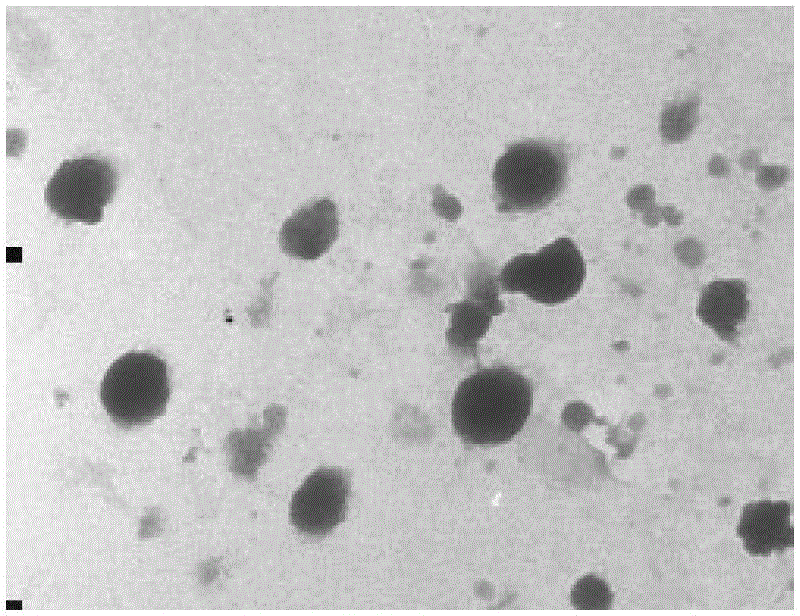
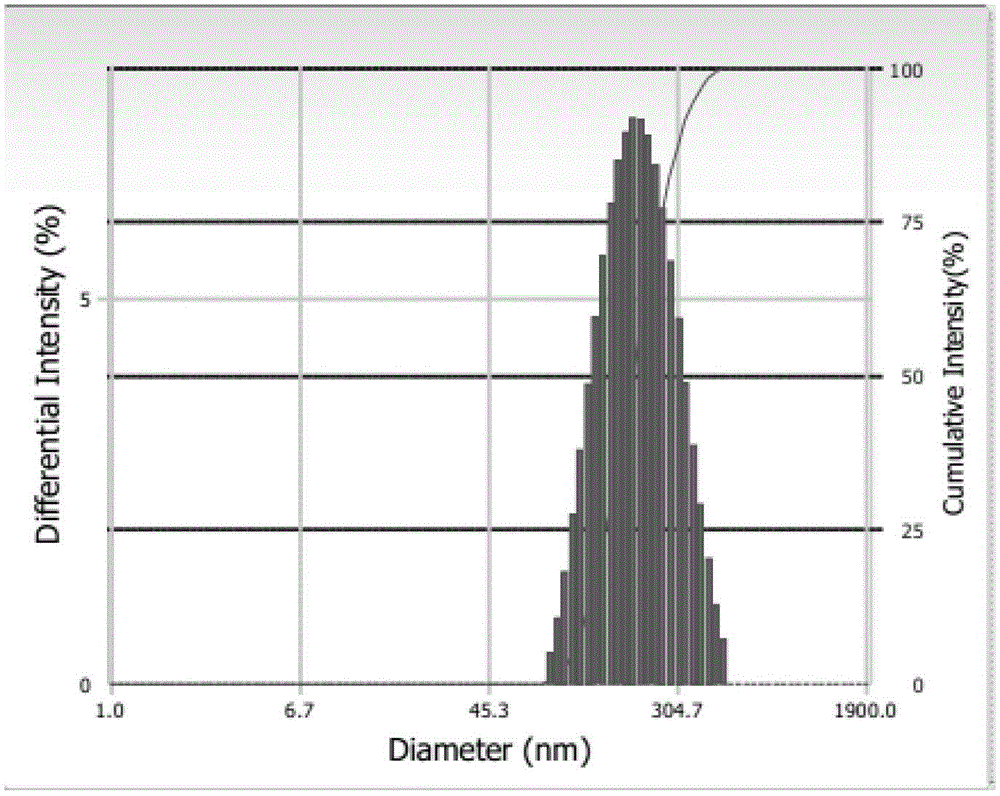
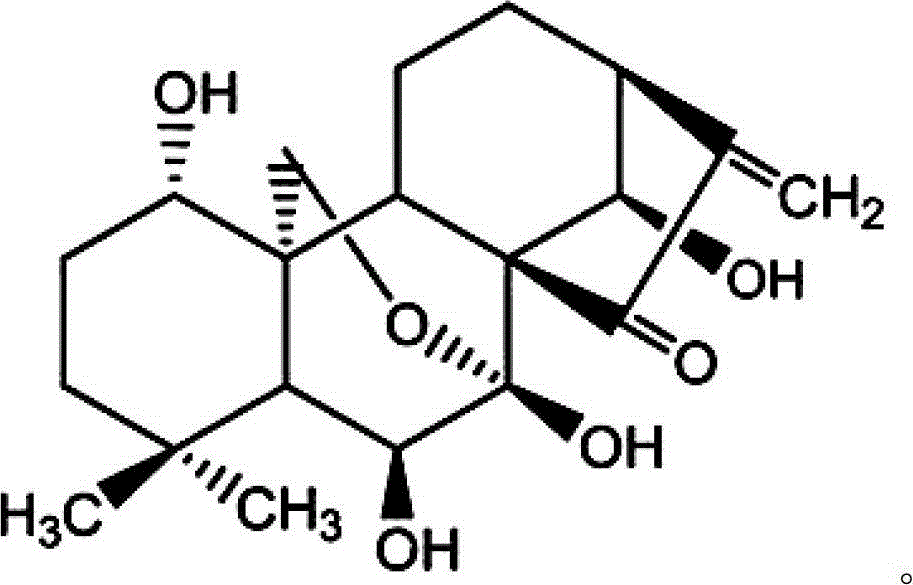
Galactose-mediated oridonin albumin nanoparticle and preparation method thereof
A technology of albumin nanoparticles and oridonin A, which is applied in the directions of medical preparations containing active ingredients, pharmaceutical formulas, powder delivery, etc., can solve problems such as difficulties in preparation methods, and achieves simple and feasible preparation methods and reduced size. Administering dosage, expanding the effect of clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of galactose-mediated oridonin albumin nanoparticles
[0039] Proceed as follows:
[0040] Dissolve 0.12g lactobionic acid in distilled water containing 0.12g EDAC and 0.06g NHS, adjust the pH to 4.5 with TMEA, adjust the pH to 7 with NaOH solution after activation for 0.5h, then add 0.08g bovine serum albumin, 500r / min After stirring for 48 hours, the resulting solution was dialyzed in a dialysis bag for 2 days. The water was changed three times a day, and then freeze-dried (dilute the dialyzed solution into vials according to 2 mL / bottle, and quickly put it in an ultra-low temperature refrigerator at -80℃ Freeze for 24 hours, then take it out and quickly put it on the shelf of the freeze dryer whose temperature has dropped to -50°C, cover the vacuum cover, turn on the vacuum pump, freeze-dry for 48 hours, take it out) to obtain galactosylated albumin.
[0041] Weigh 6.0 mg of oridonin and add it to 1.5 ml of ethanol, and dissolve oridonin under ultras...
Embodiment 2
[0043] Example 2: Preparation of galactose-mediated oridonin albumin nanoparticles
[0044] Proceed as follows:
[0045] Dissolve 0.15g lactobionic acid in distilled water containing 0.15g EDAC and 0.75g NHS, adjust the pH to 4 with TMEA, adjust the pH to 7 with NaOH solution after activation for 0.5h, then add 0.1g bovine serum albumin, 500r / min After stirring for 48 hours, the obtained solution was dialyzed in a dialysis bag for 2 days, the water was changed three times a day, and then freeze-dried to obtain galactosylated albumin.
[0046] Weigh 6.0 mg of Rubescensine A into 2.0 ml of ethanol, and dissolve Rubescensine A under ultrasound. Dissolve 100.0 mg of galactosylated albumin in 10 ml of water for injection, adjust the pH to 9 with NaOH solution, add oridonin ethanol solution drop by drop, and drop at 0.75 ml / min under magnetic stirring at 600 rpm Add absolute ethanol until nano opalescence appears, then add 1.2 mg of glutaraldehyde for chemical cross-linking for 24 hours;...
Embodiment 3
[0047] Example 3: Preparation of galactose-mediated oridonin albumin nanoparticles
[0048] Proceed as follows:
[0049] Dissolve 0.12g lactobionic acid in distilled water containing 0.12g EDAC and 0.06g NHS, adjust the pH to 5 with TMEA, adjust the pH to 7 with NaOH solution after activation for 0.5h, then add 0.08g bovine serum albumin, 500r / min After stirring for 48 hours, the obtained solution was dialyzed in a dialysis bag for 2 days, the water was changed three times a day, and then freeze-dried to obtain galactosylated albumin.
[0050] Weigh 8.0 mg of oridonin and add it to 2.0 ml of ethanol, and dissolve oridonin under ultrasound. Dissolve 75.0mg of galactosylated albumin in 5ml of water for injection, adjust the pH to 9 with NaOH solution, add oridonin ethanol solution drop by drop, and drop by 0.75ml / min under magnetic stirring at 500rpm Add absolute ethanol until nano opalescence appears, and add 1.4 mg of glutaraldehyde for chemical cross-linking for 24 hours; after cr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com