Light emitting tetraphenylene derivatives, its method for preparation and light emitting device using the same derivatives
A technology of light-emitting device and organic light-emitting device, which is applied in the direction of electroluminescent light source, light-emitting material, electric solid device, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
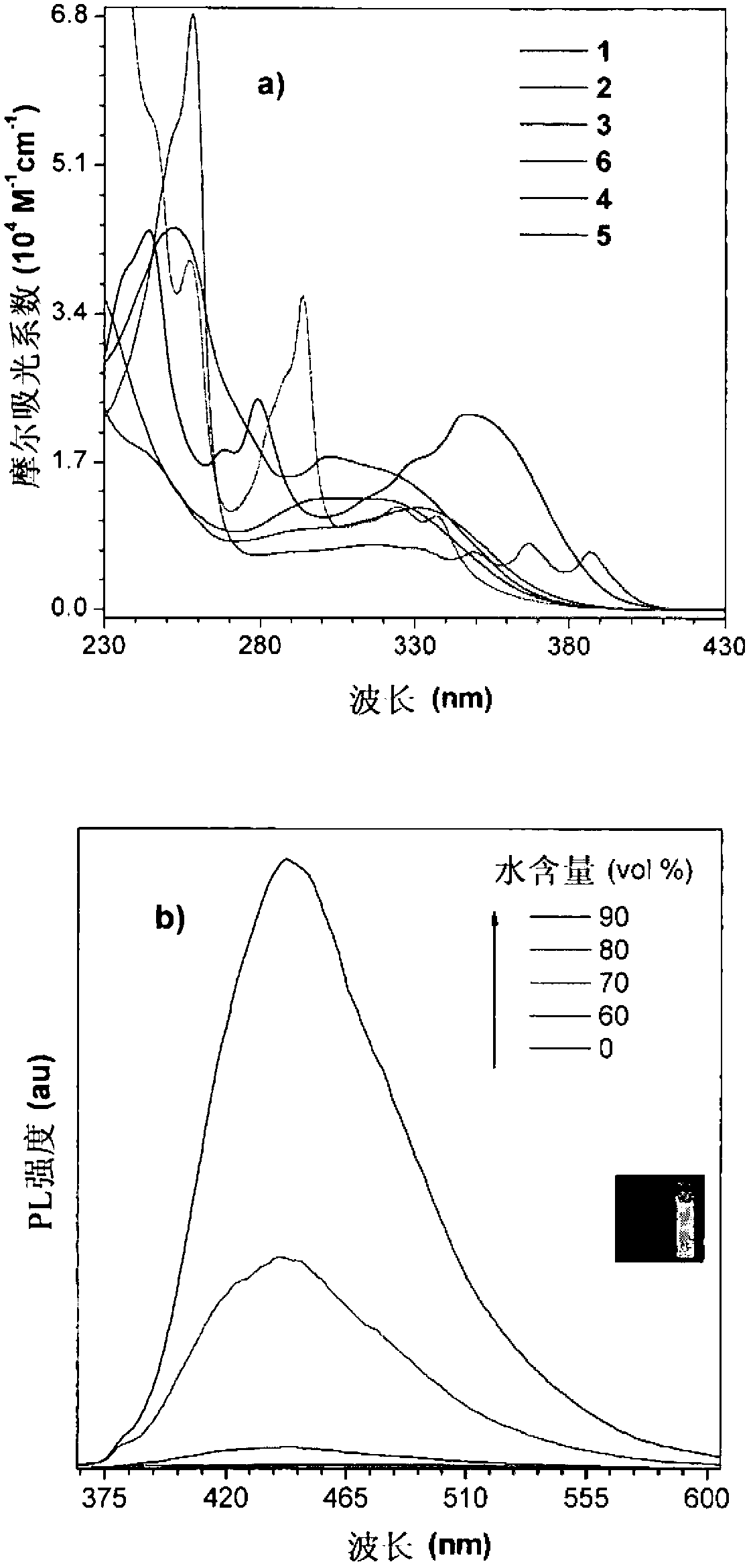
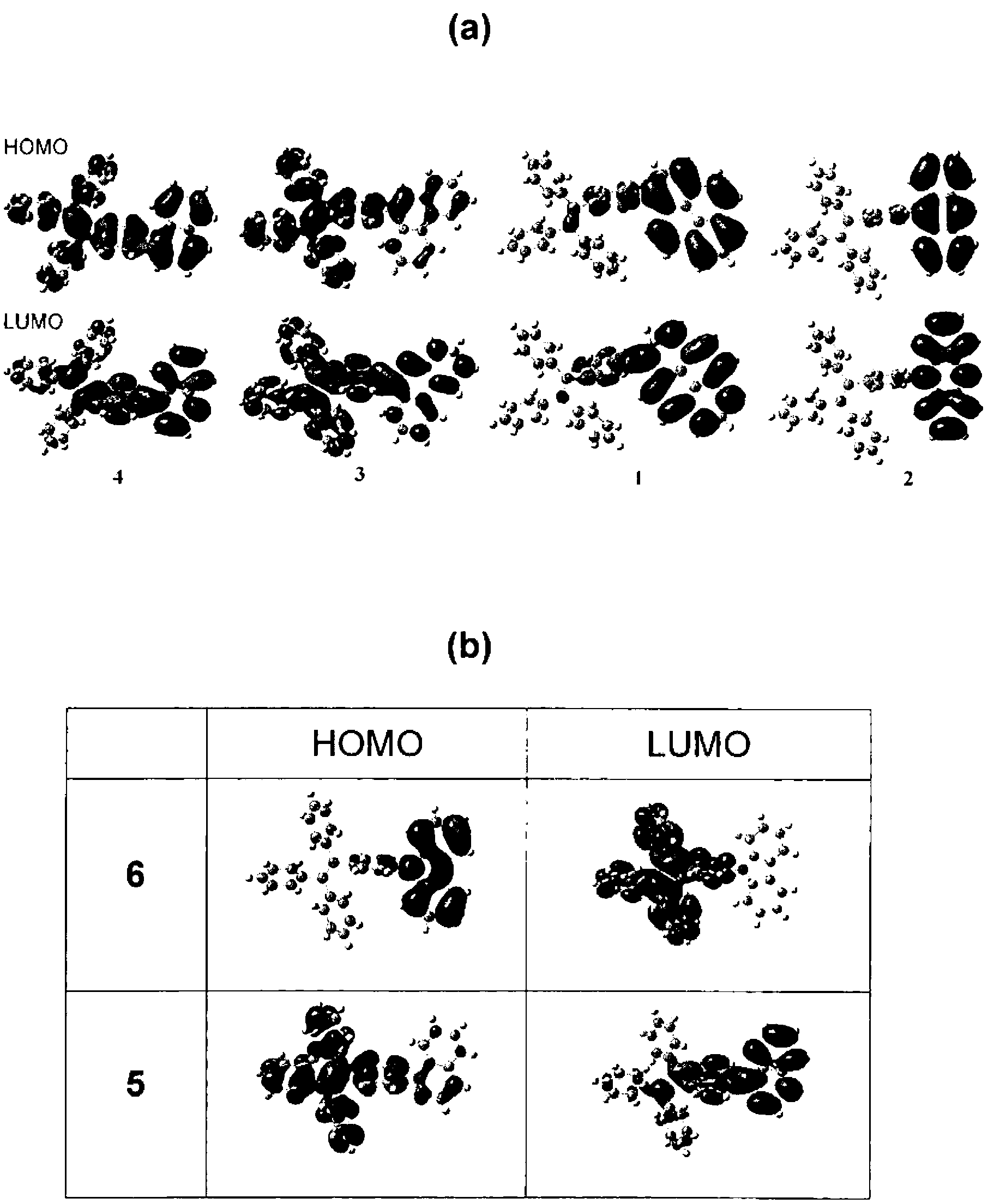
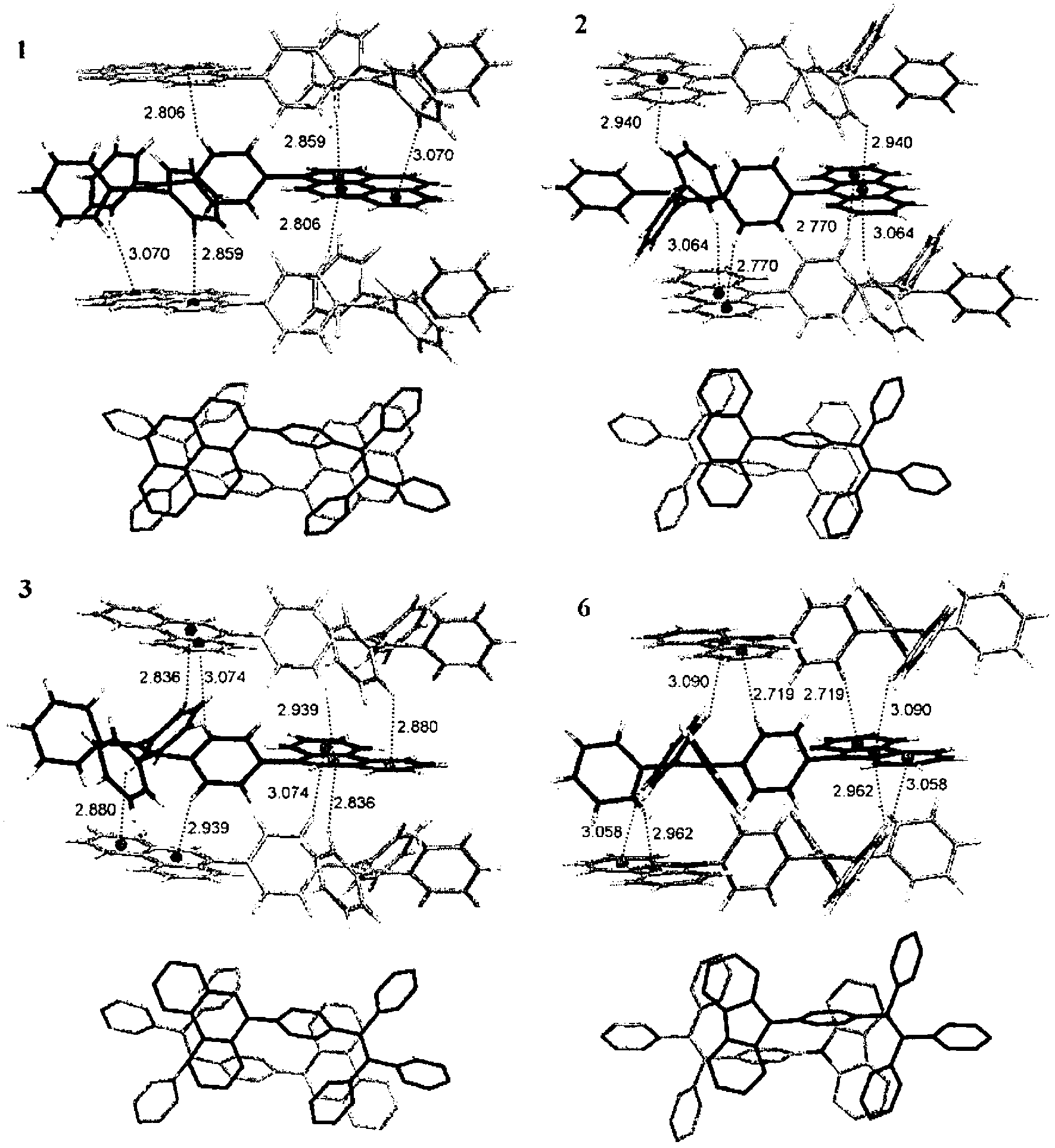
[0108] The preparation methods of these materials or molecules are simple, and all materials can be obtained in high yields as shown below. All these dye molecules have high thermal stability due to the presence of a large number of aromatic rings in the structure. These molecules fluoresce strongly in the solid state. The electroluminescence of these molecules has shown excellent results and thus can be used in organic light-emitting diodes.
[0109] In one aspect of the present invention, a luminescent material such as a dye molecule is provided, which comprises one or more tetraphenylethylene derivatives having the structural formula of compound 29 in the following scheme 1, and a preparation method of the luminescent material. Among them, R 1 , R 2 , R 3 , R 4 Each is independently selected from H and any organic or organometallic group. These materials have high solid-state quantum efficiency and thermal stability.
[0110] Schema 1
[0111]
[0112] In one emb...
example 1
[0215]
[0216] Compound 19 (1.0mmol), 1-bromopyrene (1.1mmol), Pd(PPh 3 ) 4 (0.05mmol) and potassium carbonate (4.0mmol) was dissolved in 100mL of toluene / ethanol / water (volume ratio 8:1:1), and heated to reflux for 24 hours under nitrogen protection. After filtration and evaporation of the solvent, the residue was purified by column chromatography on silica gel with hexane / dichloromethane or ethyl acetate as eluent.
[0217] Characterization data: white solid; 63% yield; m.p.: 303°C. 1 H NMR (300MHz, CD 2 Cl 2 ), δ(TMS,ppm):8.21–8.16(m,3H),8.11–7.93(m,6H),7.37(d,2H,J=8.7Hz),7.22–7.08(m,17). 13 C NMR (75MHz, CD 2 Cl 2 ),δ(TMS,ppm):144.5,144.4,144.3,143.4,142.1,141.4,139.8,138.3,132.2,131.7,131.2,130.6,129.1,128.4,128.2,128.1,128.0,127.2,1126.0,7, ,125.6,125.4,125.3.MS(MALDI-TOF):m / z532.2513(M + , calculated value 532.2191). Elemental analysis: C 42 h 28 Calculated value: C, 94.70; H, 5.30. Found value: C, 94.64; H, 5.29.
example 2
[0219]
[0220] Compound 19 (1.0mmol), 9-bromoanthracene (1.1mmol), Pd(PPh 3 ) 4 (0.05mmol) and potassium carbonate (4.0mmol) were dissolved in 100ml of toluene / ethanol / water (volume ratio 8:1:1), heated to reflux under nitrogen protection for 24 hours. After filtration and evaporation of the solvent, the residue was purified by column chromatography on silica gel with hexane / dichloromethane or ethyl acetate as eluent.
[0221] Characterization data: White solid; Yield: 69%. m.p.: 301°C. 1 H NMR (300MHz, CD 2Cl 2 ),δ(TMS,ppm):8.45(s,1H),8.03(d,2H,J=8.4Hz),7.59(d,2H,J=8.7Hz),7.48-7.43(m,2H),7.38 -7.33(m,2H),7.25-7.13(M,19H). 13 C NMR (75MHz, CD 2 Cl 2 ), δ (TMS, ppm): 144.6, 144.4, 144.2, 143.9, 142.3, 137.6, 137.5, 132.2, 132.1, 132.03, 132.00, 131.9, 131.2, 130.8, 129.0, 128.51, 128.45, 128.4, 127 ,126.0,125.9.MS(MALDI-TOF):m / z 508.2436(M + , calculated value 508.2191). Elemental analysis: C 40 h 28 Calculated value: C, 94.45; H, 5.55. Found value: C, 94.14; H,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com